1 Introduction
Binge-eating disorder (BED) is a serious psychiatric disorder with detrimental effects on physical and mental health. Patients with BED engage in recurrent episodes of eating abnormally large amounts of food in discrete periods of time while feeling a lack of control over their actions (1). Patients with BED frequently eat until they are uncomfortably full and experience elevated levels of distress around their behavior (1). The Diagnostic and Statistical Manual of Mental Disorders – 5th Edition (2) states that for a diagnosis of BED, binge eating must occur at least one day per week for three months, and the binges must not be associated with compensatory behaviors such as self-induced vomiting, excessive exercise, or fasting. BED is more common than anorexia nervosa and bulimia nervosa combined (3), with a lifetime prevalence of 1.9% in upper- and high-income countries (4, 5), however the actual prevalence of BED is likely much higher given many individuals who meet BED criteria never get a formal diagnosis or receive treatment (6). Approximately 30% of patients with BED have excess weight, and 32% are classified as having obesity (classes 1 – 3), resulting in many serious health conditions (4).
Many BED patients have secondary health complications including type II diabetes, hypertension, dyslipidemia, heart disease, and stroke (4, 7). Psychiatric comorbidities are seen in up to 80% of patients with BED, with many patients having increased rates of anxiety disorders, major depressive disorder, obsessive-compulsive disorder, and bipolar I and II (8). Patients with BED also score poorer on quality of life measures due to excess weight and higher levels of depression and anxiety (9).
The impact of BED on healthcare utilization is significant; many patients with BED receive inappropriate care through weight-loss programs and engage in treatments aimed at addressing their psychological comorbidities (4, 10) rather than their eating disorder as the root problem. The economic cost of BED is substantial; in the United States, the one-year general healthcare cost for those with BED was $18,152 higher than those without an eating disorder (in estimated 2014 USD; 11). Another study from the USA found that people with BED have higher generic healthcare costs by approximately $2,758 per year compared with their age- and gender-matched means (in estimated 2009 USD; 12). In the UK, the yearly cost of health services per eating disorder patient is £8,850, with an additional £19,700 in direct and indirect financial costs to patients and their caregivers (13). Such figures are unknown for Dutch patients and caregivers; however, these figures are likely similar in the Netherlands, highlighting the need for effective, efficient, and low-cost treatments for BED.
Cognitive behavioral therapy-enhanced (CBT-E; 14), is an evidence-based treatment for BED (15–17). CBT-E uses a transdiagnostic approach for the treatment of eating disorders and takes a highly individualized approach (12). The treatment is broken down into four stages to strategically address disturbances in eating behaviors, establish a normalized eating pattern, and promote new ways of coping (12). CBT for eating disorders can be delivered in person, through guided self-help (18–22) or self-help with minimal support and guidance from a therapist (23), video conferencing (24), or a recently developed web-based GSH program (21, 25). Given CBT has several methods of delivery, it is important to evaluate whether a stepped-care model (e.g. providing patients with low-cost and low-intensity treatment first before referring to specialized or more intensive care) is feasible, whereby less-intensive treatments such as web-based GSH are offered as a first-line of treatment (16).
The stepped-care model, or firstly referring patients for low-intensive care and increasing the level of care as needed, is ideal from an economic and healthcare perspective, as it can decrease the need for costly and specialized services, reduce treatment waiting times compared with more conventional treatments (26), and may improve long-term patient outcomes (27, 28). The stepped-care model is also preferable for patients as there is less social disruption, less or no need to take time off from school or work, geographical barriers are removed, and patients can have increased autonomy (29, 30). The stepped-care model is recommended for BED in the United Kingdom and Australia, whereby GSH is recommended as the first line of treatment (15, 16). However, despite the potential cost savings in the Netherlands (21, 31, 32) and long waiting lists for treatment, the Netherlands continues to recommend in-person therapy as a first line of treatment (33). Therefore, it is critical to examine whether a lower-intensity and remotely accessible treatments such as GSH and web-based GSH are viable for patients with BED. If GSH is offered as a first line of treatment, patients will benefit from shorter waitlists, improved health and quality of life, and the healthcare burden from BED can be mitigated (34, 35).
The first version of CBT-E self-help was developed by Christopher Fairburn (18, 36) in the book Overcoming Binge Eating, a self-help book comprised of two parts: Part I offers a description of binge eating and why it occurs, and Part II provides a CBT-E-based self-help program to stop binge eating. The book is intended for anyone with a binge-eating problem, as long as they are not significantly underweight (36). Studies have suggested GSH can empower patients to take ownership and accountability over their eating disorder recovery (37, 38), leading to a more robust recovery. Furthermore, a review on self-help and GSH for eating disorders suggests that patients using these modalities learn to manage binge eating without relying on external supports, which may better support them in the long-term to handle setbacks. If patients learn and develop the skills that they need to manage their binge eating on their own without relying on external supports, they may be better equipped to handle setbacks (37). A recent randomized controlled trial comparing web-based GSH CBT-E with a waitlist condition found that the treatment reduced the number of objective binges from an average of 19 (SD = 16) at the start-of-treatment to 3 (SD = 5) at the end of treatment, with an interaction effect between time and condition (treatment or waitlist; F2,178 = 18.55; P21). These improvements were sustained at 12- and 24-week follow-ups (21). These findings highlight the importance of not only GSH, but remotely accessible GSH treatments for BED, which could be offered to patients who lack access to treatment or do not have a formal diagnosis, While web-based GSH CBT-E offers many advantages, it is unknown whether it is as efficacious as treatment as usual CBT-E. This is critical in determining whether web-based GSH is a viable alternative that can be offered as a first line of treatment for BED.
The present study aims to determine whether web-based GSH CBT-E is noninferior in efficacy compared to treatment as usual CBT-E (TAU CBT-E) for treating BED. Efficacy will be examined by the reduction of binge-eating days, defined as the number of days on which one or more binge-eating episodes occurred, at the end of treatment. This study sought to determine whether web-based GSH CBT-E is noninferior in efficacy compared to TAU CBT-E in reducing the number of binge-eating days at the end of treatment. In addition to examining the reduction in binge-eating days, several secondary outcomes will also be evaluated, including full remission, body shape dissatisfaction, therapeutic alliance, clinical impairment, health-related quality of life, attrition, and an economic evaluation to assess the cost-effectiveness and cost-utility of web-based GSH CBT-E compared to TAU CBT-E (described below). Moderators will be examined, including baseline scores, demographic variables, and body mass index (BMI). It is expected that participants in both groups will have a reduction in the number of binge-eating days, with web-based GSH CBT-E being noninferior to TAU CBT-E.
2 Method
2.1 Trial design
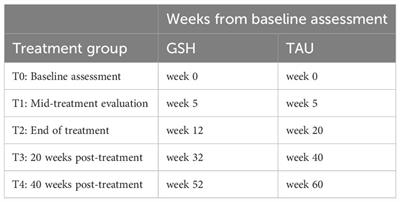
A randomized controlled trial will be conducted comparing the efficacy and noninferiority of a 12-week, web-based GSH CBT-E with the standard 20-week TAU CBT-E for BED at a specialized center for the treatment of eating disorders in the Netherlands (Novarum). Both treatments will be based on the CBT-E treatment protocol and are web-based. Assessments will take place at baseline (before randomization) and at four timepoints during and after treatment. Because the treatments vary in length, assessments will be synchronous in treatment phase rather than in the number of weeks since baseline. Web-based GSH CBT-E assessments will begin at the start of treatment (week 0), mid-treatment (week 5), end of treatment (week 12), 20 weeks post-treatment (week 32), and 40 weeks post-treatment (week 52). TAU CBT-E assessments will begin at the start of treatment (week 0), mid-treatment (week 5), end of treatment (week 20), 20 weeks post-treatment (week 40), and 40 weeks post-treatment (week 60; see Table 1 for the breakdown of assessment timing). The duration of treatment and assessments in this study is 52 weeks for GSH and 60 weeks for TAU, making the total time horizon of the study 60 weeks (14 months). The study will be performed in line with the updated CONSORT guidelines for reporting parallel group randomized trials (39). To examine the cost-effectiveness and cost-utility of the web-based GSH CBT-E and TAU CBT-E, a complete economic evaluation will be conducted using ISPOR and CHEERS guidelines (40, 41). This study has been approved by the Medical Ethical Committee-United.
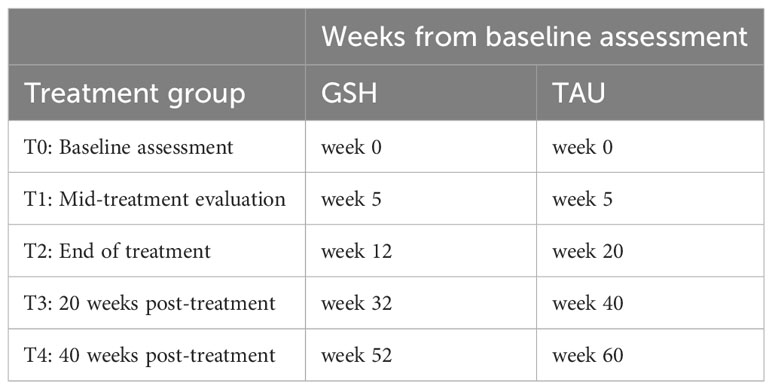
Table 1 Timing of planned assessments between groups.
2.2 Participants
2.2.1 Eligibility criteria
Participants must have a referral from their general practitioner, a DSM-5 diagnosis of BED or Other Specified Feeding and Eating Disorder – BED subtype (BED of subthreshold frequency and/or duration) as assessed by a psychiatrist or clinical psychologist, be ≥ 18 years, have a BMI between 19.5 kg/m2 ≤ 40 kg/m2, be sufficiently proficient in Dutch, have internet access and a computer or tablet, and provided informed consent to participate in the study. If a participant chooses to not participate in the study, they will be offered TAU CBT-E. Participants will be excluded if they have an eating disorder other than BED/Other Specified Feeding and Eating Disorder – BED subtype, acute psychosis, major depressive disorder, or suicidal ideation, as assessed via the Structural Clinical interview DSM-5 (SCID-5; 40, 42), have undergone any eating disorder treatment/intervention in the 6 months prior to their assessment, are pregnant, use recreational drugs (ex. marijuana, ecstasy) or take any medication that might influence eating behavior such as stimulant medications (ex. lisdexamphetamine), chlorpromazine, lithium, or olanzapine.
2.2.2 Recruitment
Participants will be recruited from a Dutch eating disorder treatment center. Eligible patients will receive written and verbal study information and an informed consent form that will explain the research goals and provide information about their participation in the study. Patients will have two weeks to decide whether they want to participate in the study, during which time they can ask any questions they have regarding the study. Once an informed consent form has been signed and received, a phone appointment will be scheduled to conduct the baseline assessment. Participants will be informed that they can discontinue the study for any reason and at any time. To promote participant retention and follow-up, participants will be encouraged to contact the lead researcher or their therapist if they have any concerns, and they will receive a €20 gift card after completion of the final posttreatment assessment (week 40).
2.3 Interventions
2.3.1 Therapists
All therapists are specialized in CBT-E treatment for eating disorders, have completed training by the Centre for Research on Eating Disorders at Oxford (CREDO), and have read Overcoming Binge Eating (36). To ensure a thorough understanding and adherence of administering web-based GSH CBT-E and TAU CBT-E, therapists will complete a two-day workshop and attend weekly 45-minute supervision sessions by the project leader (author BM) to ensure adherence to treatment plans and research protocols.
2.3.2 Treatment as usual CBT-E
Treatment as usual CBT-E is the focused version of CBT-E, as it is indicated for patients with BED and exclusively addresses eating disorder pathology and is more effective than the broad version, which addresses external mechanisms (14). During the COVID-19 pandemic, face-to-face CBT-E treatment for BED could no longer continue, and consequently, video conferencing CBT-E was introduced as TAU at Novarum to ensure continued patient care. In this study, TAU CBT-E will be delivered through video conferencing over 20 sessions, each 50 minutes in length. The treatment follows the same structure and duration as face-to-face CBT-E; however, it is offered completely remotely. Video conferencing CBT-E has been as effective as face-to-face treatment (27, 31, 43, 44). Participants receiving TAU CBT-E will be required to monitor their eating behaviors, weigh themselves regularly, evaluate their progress, find alternative activities to binge eating, improve their problem-solving skills, plan for relapse prevention, and learn ways to cope through setbacks (36).
2.3.3 Web-based guided self-help CBT-E
Web-based guided self-help CBT-E is an adapted and brief version of the focused form of CBT-E. The treatment is based on the guided self-help book Overcoming Binge Eating (36) is also available in Dutch Overwin je eetbuien, waarom je te veel eet en hoe je daar mee kunt stoppen (43), and is being built into a Dutch online application (Karify by Avinty). Before beginning web-based GSH, participants will be required to read part 1 of Overcoming Binge Eating (44). Treatment will be offered completely remotely using the web-based application, and focus on the same CBT-E principles as in TAU: monitoring eating behaviors, regular weighing, evaluating progress, finding alternative activities to binge eating, and improving problem-solving skills (36). Participants will receive weekly scripted feedback from a therapist during 12 phone sessions of 20 minutes. During these calls, therapists will review past assignments and discuss next steps in treatment.
2.4 Outcomes
2.4.1 Primary outcome
The primary outcome will be the reduction in the number of binge-eating days in the previous 28 days between baseline and the end-of-treatment between groups. Binge-eating days will be defined as days on which one or more binge-eating episodes occurred, as reported by each participant. The primary outcome will be measured at the end of treatment.
2.4.2 Secondary outcomes
Several secondary outcomes will be examined in this study, including full remission, body-shape dissatisfaction, therapeutic alliance, clinical impairment, health-related quality of life, attrition, and an economic evaluation using the costs associated with psychiatric illness, and treatment costs to assess cost-effectiveness and cost-utility, as described in detail below. The timing of secondary outcome measurements is outlined in Table 2. Baseline scores, demographic variables, and BMI will be examined as moderators.
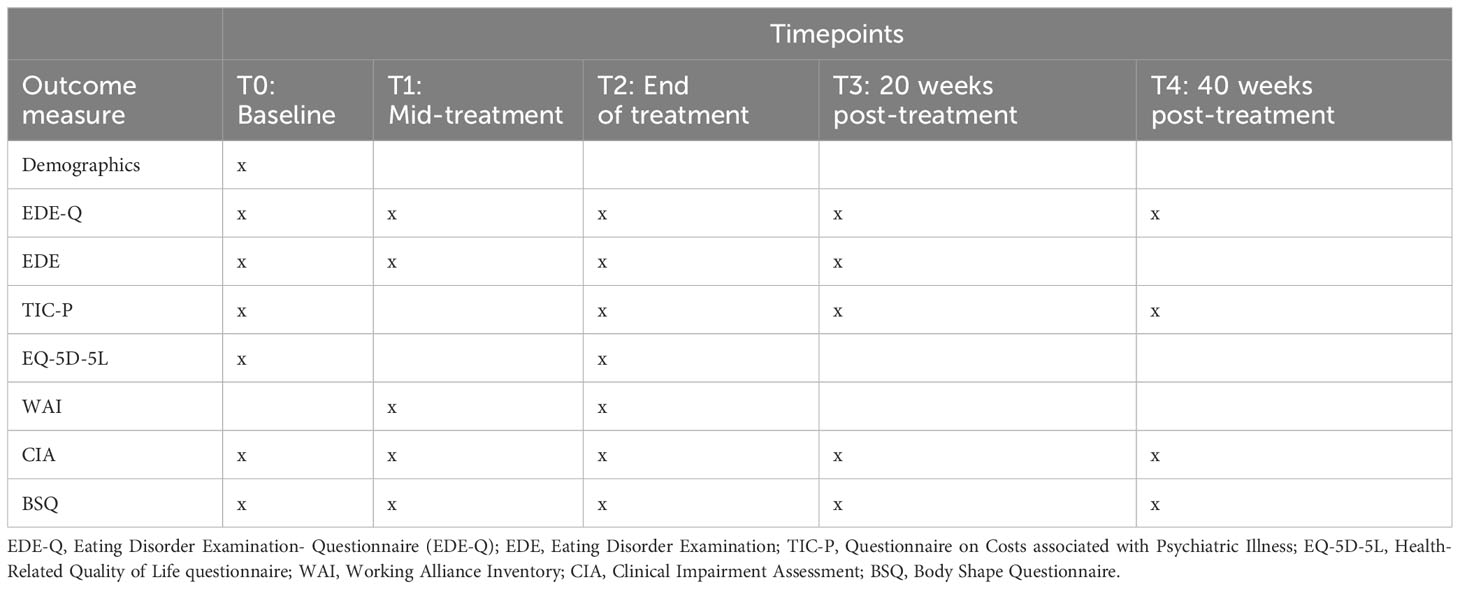
Table 2 Timing of assessments and outcome measurements.
2.4.2.1 Full remission
Full remission will be assessed using the Eating Disorder Examination (EDE; 45) interview, with a combined measure of no binge-eating episodes in the previous 28 days, and eating disorder pathology below clinical cut-off of 2.8 (46). The EDE interview is a semi-structured interview assessing eating disorder pathology during the last 28 days, including binge eating. The scale consists of four subscales on dietary restraint, eating concern, weight concern and shape concern, and is measured on a 7-point Likert scale (47). The EDE has good internal consistency, discriminative validity, and reliability (48), and has been translated and validated in Dutch (49). The Eating Disorders Examination Questionnaire (EDE-Q; 50, 51) will be used at the 40-week post-treatment assessment. The EDE-Q is a 36-item questionnaire based on the EDE and the global score is calculated as the sum of the four subscales scores, divided by four (the number of subscales).
2.4.2.2 Body shape dissatisfaction
Body shape dissatisfaction will be examined using the Body Shape Questionnaire (BSQ; 47). The BSQ contains 34 questions on a 6-point Likert scale ranging from “never” to “always”. The questionnaire has demonstrated good concurrent and discriminant validity, internal consistency, and test-retest reliability (52, 53).
2.4.2.3 Therapeutic alliance
Therapeutic alliance, or the working relationship a patient has with a therapist, will be measured using Dutch version of the Working Alliance Inventory (WAI; 54, 55) to examine differences in therapeutic alliance between GSH and TAU, given therapeutic alliance can be impacted in online therapy (56). The WAI is a self-reported questionnaire with 36 items scored on a 7-point Likert scale examining the alliance between the patient and therapist. For the purposes of this study, only the patient’s perspective will be assessed. The questionnaire has three dimensions: Bonds, Tasks, and Goals. The Dutch version has been validated by (55).
2.4.2.4 Clinical impairment
Clinical impairment refers to the psychosocial impairment suffered by patients as a direct result of their binge-eating disorder (Fairburn, 2008). The Clinical Impairment Assessment (CIA; 57) will be used to examine impairment secondary to eating disorder psychopathology, as the CIA differentiates between eating disorder pathology and secondary impairment due to the eating disorder. The questionnaire is self-reported and consists of 16 items with personal, social, and cognitive subscales, and is rated on a 4-point Likert scale.
2.4.2.5 Health-related quality of life
Health-related quality of life will be measured using the Dutch version of the Euroqol EQ-5D-5L, a five-dimension, five level measure of health status (58). The scale has five dimensions with one item per dimension on mobility, self-care, usual activities, pain and discomfort, and anxiety/depression. Each item is rated on a 5-point scale with: no problems, slight problems, moderate problems, severe problems and extreme problems.
2.4.2.6 Attrition
Attrition bias will be measured to determine whether participants in either group are more likely to have incomplete assessments or prematurely dropout of treatment. Attrition will be measured by the total amount of non-response and missing data (59), and dropout will be measured by the total number of participants that formally withdraw from treatment.
2.4.2.7 Cost associated with a psychiatric illness
The costs associated with psychiatric illness will be measured using the Dutch version (60) of the questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P) (61). The questionnaire for adults is composed of three parts and considers the cost of medical and mental health care, company doctors, social services, allied health care, residential care, alternative care, home help and support, addiction care, medication, and diet. The TiC-P will be used for the cost-effectiveness and cost-utility analysis to measure health care use and productivity loss.
2.4.2.8 Cost of treatment
The cost of treatment will be determined from a healthcare perspective. For each patient, the cost of treatment will be calculated by multiplying standard Dutch cost prices (62) by the total number of minutes spent on direct patient contact. The time horizon for this study will be 52 and 60 weeks; therefore, no discounting for future costs will be applied.
2.4.2.9 Cost-effectiveness and cost-utility
A cost-effectiveness analysis will be performed using the reduction in number of binge-eating days in the previous 28 days. A cost-utility analysis will examine the number of quality-adjusted life-years (QALYs) gained between randomization and post-treatment (63). Quality of life will be measured using the Dutch three-level variant of the EQ-5D (58). The Dutch tariff (64) will be used to translate EQ-5D-5L scores into health utilities, and utility weights will be assigned which reflect the patient’s health between death (0) and perfect health (1) (65). One QALY reflects one year in perfect health. The incremental cost-effectiveness ratio (ICER) will be calculated using the costs and effects of treatment. Confidence intervals of the ICER will be calculated using non-parametric bootstraps to provide incremental costs, effects, and a cost-effectiveness ratio. Data on resource use (health care uptake) and productivity losses will be collected with a Dutch version of the TiC-P (60, 61).
2.4.3 Moderators
Baseline scores, demographic variables, and BMI will be examined as moderators. Baseline scores, including the number of objective binges and eating disorder severity will be examined given prior research suggests that more severe BED pathology at baseline predicts less improvement in BED pathology at the end of CBT-E treatment (66, 67), and may also predict greater treatment dropout (21). Demographics, such as age, gender, and level of education, will be examined as possible moderators. Lastly, BMI, calculated by dividing the participant’s weight in kilograms by the square of height in meters (kg/m2), will be examined as a moderator.
2.5 Statistics
2.5.1 Sample size and power estimation
The primary objective of the proposed study is to determine whether GSH is noninferior to TAU in efficacy. It is expected that both groups will have a reduction in the number of binge-eating days, and GSH is expected to be noninferior compared to TAU in the reduction of binge-eating days in the previous 28 days at the end of treatment.
To calculate the sample size for this noninferiority trial, the significance level was set at α = 0.05, power was aimed at 80%, and the noninferiority margin (Δ) was set to 1 (SD = 2.4) binge-eating day (68) in the previous 28 days between groups. With these assumptions, n = 144 (n = 72 per arm) participants will be needed to determine noninferiority. The noninferiority margin and standard deviation are based on noninferiority margins from prior studies examining online treatments to reduce eating disorder behaviors (68, 69) and clinical experts on BED treatment outcomes. Efficacy studies of CBT-E (70) and internet-based guided self-help for BED (68) report a 20% dropout; therefore, 20% more participants will be included to compensate for dropout. The sample size corrected for dropout is N = 180 (n = 90 per arm); therefore 180 participants will be randomized. This sample size calculation was performed using the R package ‘SampleSize4ClinicalTrials’ (71–73) based on a continuous outcome, noninferiority design.
2.5.2 Randomization
Randomization will be performed using a 4, 6, 8 block design by Castor Electronic Data Capture (Amsterdam, the Netherlands, 2019) and will take place after the baseline assessment. The allocation ratio will be 1:1. This software will ensure the concealment of allocation and blinding. Stratification will take place based on a BMI of 19.5 ≤ 35 or 35.1 ≤ 40.
2.5.3 Statistical analyses
To measure the efficacy of treatment on the reduction of binge-eating days, the number of binge-eating days will be reported as means (standard deviation) between groups and analyzed using a general linear mixed model. Effect sizes will be calculated using Cohen’s d (0.2 small, 0.5 medium, 0.8 large; 74) and adjusted for bias (75). Sensitivity analyses will be conducted to test the robustness of the results; given the 20% anticipated dropout rate, analyses will be run on an intention-to-treat protocol, and multiple imputations will be used to handle missing data. To examine possible treatment moderators, a regression analysis with subgroups will be performed by analyzing interaction terms between the moderators and treatment group. Data will be nested within BMI groups (19.5 ≤ 35 and 35.1 ≤ 40) and include random and fixed effects. If significant interactions exist, post-hoc analyses will be performed to further explore the moderation.
The cost-effectiveness and cost-utility analyses will be performed from a healthcare perspective. The cost-effectiveness analysis will examine the reduction in binge-eating days as the measure of effect measure, and the cost-utility analysis will use QALYs as effect measure. Differences in costs and effects between groups will be calculated as the difference in cumulative direct costs using the reduction of binge-eating days as the outcome measure. ICERs will be calculated as ICER = (Costs GSH − Costs TAU)/(Effects GSH – Effects TAU) with reduction in binge-eating days as the effect. ICERs to report comparative differences in QALYs will be calculated as ICER = (Costs GSH − Costs TAU)/(Effects GSH – Effects TAU). The costs, effects, and ICERs will be plotted on a cost-effectiveness plane to demonstrate the differences between the costs and effects of both groups, with TAU positioned at the origin of the cost-effectiveness plane. Cost-effectiveness acceptability curves will be used to examine the probability that the cost-effectiveness of GSH is equally as effective with lower costs compared to TAU by a willingness-to-pay for each unit of effect (reduction in binge-eating days or QALYs). The willingness-to-pay for each additional unit of effect in the Netherlands ranges between €20,000 and €80,000 per QALY (25, 76) and €22–€110 per binge-free day in the United Kingdom and the United States (31). All statistical analyses will be conducted using R (77) and SPSS (78).
2.5.4 Data monitoring
All data will be stored in the secure data storage environment within the primary institute and will only be accessible to relevant senior researchers and the project leader with secure login procedures. All data will be handled confidentially, and each participant will be provided with an identification code to separate their personal information from their data, in line with the EU General Data Protection Regulation (79) and the Dutch Act on Implementation of the General Data Protection Regulation (Uitvoeringswet AVG, UAVG). Data will be monitored by a statistician and data steward independent from the study, who will review the data collected to ensure the validity of the study findings. Any significant harmful events or reactions possibly relating to the intervention will lead to unblinding. The study will be temporarily halted if there is sufficient ground to suggest that continuing the study could jeopardize the health or safety of a participant. Any participant exhibiting a psychiatric crisis will be assessed and treated accordingly, and any serious adverse events will be reported to the METC. Finally, posttrial support will be offered to participants who require it.
3 Anticipated results
It is expected that at the end of treatment, the difference in reduction of binge-eating days between web-based GSH CBT-E and TAU CBT-E is within the noninferiority margin (Δ) of 1 binge-eating day (68). Regarding secondary outcomes, it is expected that there will be no difference in the rate of full remission, clinical impairment, or reduction in body shape dissatisfaction between groups. However, it is expected that more severe binge-eating pathology at the start of treatment will predict a weaker treatment response, regardless of condition (80). In addition, the economic evaluation is expected to demonstrate that GSH is more cost-effective than TAU, while having similar cost-utility (31, 81). Therapeutic alliance is expected to be the similar between groups (82). Attrition rate is expected to be approximately 20% in both conditions (68, 83). As for moderators, it is expected that baseline scores will moderate the treatment effect; for example, it is expected that participants with a greater number of objective binges at baseline will demonstrate a slower response to treatment (84). Demographic information is expected to have no effect on the reduction of binge-eating days. Lastly, is expected that BMI will have a small moderating effect on binge-eating reduction, such that participants with a greater baseline BMI will have a weaker response to treatment, regardless of group.
4 Discussion
This protocol outlines a randomized controlled trial comparing clinical and economic outcomes between two web-based treatments for BED: web-based GSH CBT-E and TAU CBT-E. This study will be the first to directly compare the efficacy of a low-intensity and low-cost alternative compared to TAU CBT-E alongside a complete economic evaluation. Furthermore, this study will establish whether the improvements seen in 20 weeks of TAU CBT-E can be achieved in 12 weeks of web-based GSH CBT-E. The aim of the proposed study is to determine whether web-based GSH CBT-E is noninferior to TAU CBT-E in reducing the number of binge-eating days at the end of treatment. Additional outcomes including full remission, body shape dissatisfaction, therapeutic alliance, clinical impairment, health-related quality of life, attrition, and an economic evaluation to assess the cost-effectiveness and cost-utility of web-based GSH CBT-E and TAU CBT-E will be evaluated to provide a thorough understanding of the efficacy and effectiveness of web-based GSH CBT-E and for whom it is best indicated. Baseline scores, demographic variables, and BMI will be examined as moderators to support the development of a personalized approach to treatment and to optimize the allocation of treatment resources.
The findings from this study will offer compelling insight into whether BED can be treated using a stepped-care model, whereby web-based GSH CBT-E is offered as the recommended first line of treatment for patients in the Netherlands. In this model, if a patient does not improve after web-based GSH CBT-E, they will be offered the higher intensity video conferencing CBT-E. If web-based GSH CBT-E is noninferior to TAU CBT-E in reducing the number of binge-eating days, the treatment can be widely disseminated among allied healthcare workers who identify a patient with BED. This will allow more patients to receive timely support and shorten treatment waitlists, ultimately supporting more patients in their BED recovery.
Ethics statement
The study has been approved by the Medical Research Ethics Committees United on May 25th, 2021, case number NL76368.100.21. The participants will provide their written informed consent to participate.
Author contributions
EvB: Writing – original draft. BM: Writing – review & editing. MdJ: Writing – review & editing. JP: Writing – review & editing. EvdB: Writing – review & editing. EdB: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this study has been provided by an anonymous family foundation. The funding provided by the anonymous private family foundation has no conflict of interest in this research. The foundation was not involved in the design, implementation, or presentation of this work, nor are they involved with any of the author affiliations, the institution the proposed study will be conducted at, or with the Dutch Application that is being created based on the treatment being tested.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Association AP. Diagnostic and statistical manual of mental disorders : DSM-5™ 5th edition. Washington, DC: American Psychiatric Publishing, a division of American Psychiatric Association (2013).
Google Scholar
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 Arlington, VA: American Psychiatric Association (2013).
Google Scholar
4. Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry (2013) 73:904–14. doi: 10.1016/j.biopsych.2012.11.020
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Preti A, Girolamo GD, Vilagut G, Alonso J, Graaf RD, Bruffaerts R, et al. The epidemiology of eating disorders in six European countries: Results of the ESEMeD-WMH project. J Psychiatr Res (2009) 43:1125–32. doi: 10.1016/j.jpsychires.2009.04.003
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Bray B, Bray C, Bradley R, Zwickey H. Binge eating disorder is a social justice issue: A cross-sectional mixed-methods study of binge eating disorder experts’ Opinions. Int J Environ Res Public Health (2022) 19(10):6243. doi: 10.3390/ijerph19106243
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Olguin P, Fuentes M, Gabler G, Guerdjikova AI, Keck PE, McElroy SL. Medical comorbidity of binge eating disorder. Eat Weight Disord (2017) 22:13–26. doi: 10.1007/s40519-017-0398-5
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Bray B, Bray C, Bradley R, Zwickey H. Mental health aspects of binge eating disorder: A cross-sectional mixed-methods study of binge eating disorder experts’ perspectives. Front Psychiatry (2022) 13:953203. doi: 10.3389/fpsyt.2022.953203
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Ágh T, Kovács G, Pawaskar M, Supina D, Inotai A, Vokó Z. Epidemiology, health-related quality of life and economic burden of binge eating disorder: a systematic literature review. Eat Weight Disord (2015) 20:1–12. doi: 10.1007/s40519-014-0173-9
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Mond JM, Hay PJ, Rodgers B, Owen C. Health service utilization for eating disorders: findings from a community-based study. Int J Eat Disord (2007) 40:399–408. doi: 10.1002/eat.20382
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Bellows BK, DuVall SL, Kamauu AW, Supina D, Babcock T, LaFleur J. Healthcare costs and resource utilization of patients with binge-eating disorder and eating disorder not otherwise specified in the Department of Veterans Affairs. Int J Eating Disord (2015) 48:1082–91. doi: 10.1002/eat.22427
CrossRef Full Text | Google Scholar
12. Grenon R, Tasca GA, Cwinn E, Coyle D, Sumner A, Gick M, et al. Depressive symptoms are associated with medication use and lower health-related quality of life in overweight women with binge eating disorder. Womens Health Issues (2010) 20:435–40. doi: 10.1016/j.whi.2010.07.004
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Stuhldreher N, Wild B, König HH, Konnopka A, Zipfel S, Herzog W. Determinants of direct and indirect costs in anorexia nervosa. Int J Eat Disord (2015) 48:139–46. doi: 10.1002/eat.22274
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Fairburn CG. Cognitive behavior therapy and eating disorders Vol. xii. . New York, NY, US: Guilford Press (2008) p. 324–xii. p.
Google Scholar
15. Hay P, Chinn D, Forbes D, Madden S, Newton R, Sugenor L, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of eating disorders. Aust New Z J Psychiatry (2014) 48:977–1008. doi: 10.1177/0004867414555814
CrossRef Full Text | Google Scholar
16. National Institute for Health and Care Excellence (NICE). National Institute for Health and Care Excellence: Guidelines. London, United Kingdom. (2017).
Google Scholar
17. Melisse B, Dekker J, van den Berg E, de Jonge M, van Furth EF, Peen J, et al. Comparing the effectiveness and predictors of cognitive behavioural therapy-enhanced between patients with various eating disorder diagnoses: a naturalistic study. Cogn Behav Therapist (2022) 15:e20. doi: 10.1017/S1754470X22000174
CrossRef Full Text | Google Scholar
18. Carter JC, Fairburn CG. Cognitive-behavioral self-help for binge eating disorder: a controlled effectiveness study. J Consult Clin Psychol (1998) 66:616–23. doi: 10.1037//0022-006x.66.4.616
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behav Res Ther (2005) 43:1509–25. doi: 10.1016/j.brat.2004.11.010
PubMed Abstract | CrossRef Full Text | Google Scholar
20. van den Berg E, Melisse B, Koenders J, de Jonge M, Blankers M, de Beurs E, et al. Online cognitive behavioral therapy enhanced for binge eating disorder: study protocol for a randomized controlled trial. BMC Psychiatry (2020) 20:190. doi: 10.1186/s12888-020-02604-1
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Melisse B, Berg E, Jonge Md, Blankers M, Furth Ev, Dekker J, et al. Efficacy of web-based, guided self-help cognitive behavioral therapy–enhanced for binge eating disorder: randomized controlled trial. J Med Internet Res (2023) 25:e40472. doi: 10.2196/40472
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Melisse B, van den Berg E, de Beurs E. Effectiveness of web-based guided self-help cognitive behavioral therapy-enhanced for binge-eating disorder: An implementation study. Int J Eat Disord (2023) 1–11. doi: 10.1002/eat.24079
CrossRef Full Text | Google Scholar
23. Striegel-Moore RH, Wilson GT, DeBar L, Perrin N, Lynch F, Rosselli F, et al. Cognitive behavioral guided self-help for the treatment of recurrent binge eating. J Consult Clin Psychol (2010) 78:312–21. doi: 10.1037/a0018982
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Abrahamsson N, Ahlund L, Ahrin E, Alfonsson S. Video-based CBT-E improves eating patterns in obese patients with eating disorder: A single case multiple baseline study. J Behav Ther Exp Psychiatry (2018) 61:104–12. doi: 10.1016/j.jbtep.2018.06.010
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Melisse B, Blankers M, van den Berg E, de Jonge M, Lommerse N, van Furth E, et al. Economic evaluation of web-based guided self-help cognitive behavioral therapy-enhanced for binge-eating disorder compared to a waiting list: A randomized controlled trial. Int J Eating Disord (2023) 56(9):1772–84. doi: 10.1002/eat.2400
CrossRef Full Text | Google Scholar
26. Shafran R, Clark DM, Fairburn CG, Arntz A, Barlow DH, Ehlers A, et al. Mind the gap: Improving the dissemination of CBT. Behav Res Ther (2009) 47:902–9. doi: 10.1016/j.brat.2009.07.003
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Crow SJ, Agras WS, Halmi KA, Fairburn CG, Mitchell JE, Nyman JA. A cost effectiveness analysis of stepped care treatment for bulimia nervosa. Int J Eating Disord (2013) 46:302–7. doi: 10.1002/eat.22087
CrossRef Full Text | Google Scholar
28. Mitchell JE, Agras S, Crow S, Halmi K, Fairburn CG, Bryson S, et al. Stepped care and cognitive–behavioural therapy for bulimia nervosa: randomised trial. Br J Psychiatry (2011) 198:391–7. doi: 10.1192/bjp.bp.110.082172
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Linardon J, Messer M, Lee S, Rosato J. Perspectives of e-health interventions for treating and preventing eating disorders: descriptive study of perceived advantages and barriers, help-seeking intentions, and preferred functionality. Eat Weight Disord (2021) 26:1097–109. doi: 10.1007/s40519-020-01005-3
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Plateau CR, Brookes FA, Pugh M. Guided recovery: an interpretative phenomenological analysis of service users’ Experiences of guided self-help for bulimic and binge eating disorders. Cogn Behav Practice (2018) 25:310–8. doi: 10.1016/j.cbpra.2017.08.004
CrossRef Full Text | Google Scholar
31. Jenkins PE, Luck A, Violato M, Robinson C, Fairburn CG. Clinical and cost-effectiveness of two ways of delivering guided self-help for people with an eating disorder: A multi-arm randomized controlled trial. Int J Eating Disord (2021) 54:1224–37. doi: 10.1002/eat.23554
CrossRef Full Text | Google Scholar
32. König H-H, Bleibler F, Friederich H-C, Herpertz S, Lam T, Mayr A, et al. Economic evaluation of cognitive behavioral therapy and Internet-based guided self-help for binge-eating disorder. Int J Eating Disord (2018) 51:155–64. doi: 10.1002/eat.22822
CrossRef Full Text | Google Scholar
33. Standaarden GGZ. (2017). Kwaliteitsstandaarden voor de geestelijke gezondheidszorg.
Google Scholar
34. Grilo CM, Masheb RM, Wilson GT, Gueorguieva R, White MA. Cognitive-behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: a randomized controlled trial. J Consult Clin Psychol (2011) 79:675–85. doi: 10.1037/a0025049
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Linardon J, Wade TD, de la Piedad Garcia X, Brennan L. The efficacy of cognitive-behavioral therapy for eating disorders: A systematic review and meta-analysis. J Consult Clin Psychol (2017) 85:1080–94. doi: 10.1037/ccp0000245
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Fairburn CG. Overcoming binge eating : the proven program to learn why you binge and how you can stop 2nd ed. New York: The Guilford Press (2013). p. 243.
Google Scholar
37. Perkins SS, Murphy RR, Schmidt UU, Williams C. Self-help and guided self-help for eating disorders. Cochrane Database Systematic Rev (2006). doi: 10.1002/14651858.CD004191.pub2
CrossRef Full Text | Google Scholar
38. Yim SH, Schmidt U. Experiences of computer-based and conventional self-help interventions for eating disorders: A systematic review and meta-synthesis of qualitative research. Int J Eating Disord (2019) 52:1108–24. doi: 10.1002/eat.23142
CrossRef Full Text | Google Scholar
39. Schulz S, Laessle RG. Associations of negative affect and eating behaviour in obese women with and without binge eating disorder. Eat Weight Disord (2010) 15:e287–93. doi: 10.1007/BF03325311
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Clin Ther (2022) 44:158–68. doi: 10.1016/j.jval.2021.11.1351
PubMed Abstract | CrossRef Full Text | Google Scholar
42. First MB, Williams JBW, Karg RS, Spitzer RL, American Psychiatric Association Publishing. User’s guide for the SCID-5-CV structured clinical interview for DSM-5 disorders : clinician version Vol. xii. . Arlington, VA: American Psychiatric Association Publishing (2016). p. 158. p.
Google Scholar
43. Mitchell JE, Crosby RD, Wonderlich SA, Crow S, Lancaster K, Simonich H, et al. A randomized trial comparing the efficacy of cognitive–behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther (2008) 46:581–92. doi: 10.1016/j.brat.2008.02.004
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Sockalingam S, Cassin SE, Wnuk S, Du C, Jackson T, Hawa R, et al. A pilot study on telephone cognitive behavioral therapy for patients six-months post-bariatric surgery. Obes Surg (2017) 27:670–5. doi: 10.1007/s11695-016-2322-x
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Fairburn C. Overwin je eetbuien Nieuwezijds BV. Amsterdam, the Netherlands (2016).
Google Scholar
46. Fairburn C. Overcoming Binge Eating. New York, USA: The Guilford Press (1995).
Google Scholar
47. Cooper Z, Fairburn C. The eating disorder examination: A semi-structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eating Disord (1987) 6:1–8. doi: 10.1002/1098-108X(198701)6:13.0.CO;2-9
CrossRef Full Text | Google Scholar
48. Turner H, Marshall E, Stopa L, Waller G. Cognitive-behavioural therapy for outpatients with eating disorders: Effectiveness for a transdiagnostic group in a routine clinical setting. Behav Res Ther (2015) 68:70–5. doi: 10.1016/j.brat.2015.03.001
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Cooper PJ, Taylor MJ, Cooper Z, Fairbum CG. The development and validation of the Body Shape Questionnaire. Int J Eating Disord (1987) 6:485–94. doi: 10.1002/1098-108X(198707)6:43.0.CO;2-O
CrossRef Full Text | Google Scholar
50. Rosen JC, Vara L, Wendt S, Leitenberg H. Validity studies of the eating disorder examination. Int J Eating Disord (1990) 9:519–28. doi: 10.1002/1098-108X(199009)9:53.0.CO;2-K
CrossRef Full Text | Google Scholar
51. Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? Int J eating Disord (1994) 16:363–70.
Google Scholar
52. Jansen A, Fairburn CG ZC. Eating disorder examination (EDE 12.0): interview ter vaststelling van de specifieke psychopathologie van eetstoornissen. Lisse, The Netherlands: Swets Test Publishers (2000).
Google Scholar
53. Lentillon-Kaestner V, Berchtold A, Rousseau A, Ferrand C. Validity and reliability of the French versions of the body shape questionnaire. J Pers Assess (2014) 96:471–7. doi: 10.1080/00223891.2013.843537
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Hatcher RL, Gillaspy JA. Development and validation of a revised short version of the Working Alliance Inventory. Psychother Res (2006) 16:12–25. doi: 10.1080/10503300500352500
CrossRef Full Text | Google Scholar
55. Paap D, Schrier E, Dijkstra PU. Development and validation of the Working Alliance Inventory Dutch version for use in rehabilitation setting. Physiotherapy Theory Practice (2019) 35:1292–303. doi: 10.1080/09593985.2018.1471112
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Kaiser J, Hanschmidt F, Kersting A. The association between therapeutic alliance and outcome in internet-based psychological interventions: a meta-analysis. Comput Hum Behavior (2021) 114:106512. doi: 10.1016/j.chb.2020.106512
CrossRef Full Text | Google Scholar
57. Bohn K, Doll HA, Cooper Z, O’Connor M, Palmer RL, Fairburn CG. The measurement of impairment due to eating disorder psychopathology. Behav Res Ther (2008) 46:1105–10. doi: 10.1016/j.brat.2008.06.012
PubMed Abstract | CrossRef Full Text | Google Scholar
58. EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands) (1990) 16(3):199–208. doi: 10.1016/0168-8510(90)90421-9
PubMed Abstract | CrossRef Full Text | Google Scholar
59. Hewitt CE, Kumaravel B, Dumville JC, Torgerson DJ, group Tas. Assessing the impact of attrition in randomized controlled trials. J Clin Epidemiol (2010) 63:1264–70. doi: 10.1016/j.jclinepi.2010.01.010
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Hakkaart-van Roijen L, Straten Av, Tiemens B, Donker M. Handleiding Trimbos/iMTA questionnaire for Costs associated with Psychiatric illness (TiC-P). Rotterdam, the Netherlands: Institute of Medical Technology Assessment (iMTA) (2002).
Google Scholar
61. Bouwmans C, De Jong K, Timman R, Zijlstra-Vlasveld M, der Feltz-Cornelis V, Tan SS, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv Res (2013) 13:1–9. doi: 10.1186/1472-6963-13-217
PubMed Abstract | CrossRef Full Text | Google Scholar
62. Zorginsituut N. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg [Guideline for economic evaluations in health care]. Diemen, the Netherlands. (2016).
Google Scholar
63. Duru G, Auray JP, Béresniak A, Lamure M, Paine A, Nicoloyannis N. Limitations of the methods used for calculating quality-adjusted life-year values. Pharmacoeconomics (2002) 20:463–73. doi: 10.2165/00019053-200220070-00004
PubMed Abstract | CrossRef Full Text | Google Scholar
64. Lamers L, Stalmeier P, McDonnell J, Krabbe P, van Busschbach J. Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff. Nederlands tijdschrift voor geneeskunde (2005) 149:1574–8.
PubMed Abstract | Google Scholar
66. Anderson LM, Smith KM, Schaefer LM, Crosby RD, Cao L, Engel SG, et al. Predictors and moderators of treatment outcome in a randomized clinical trial for binge-eating disorder. J Consulting Clin Psychol (2020) 88:631. doi: 10.1037/ccp0000503
CrossRef Full Text | Google Scholar
67. Grilo CM, Thompson-Brenner H, Shingleton RM, Thompson DR, Franko DL. Clinical moderators and predictors of cognitive-behavioral therapy by guided-self-help versus therapist-led for binge-eating disorder: Analysis of aggregated clinical trials. Int J Eating Disord (2021) 54:1875–80. doi: 10.1002/eat.23601
CrossRef Full Text | Google Scholar
68. de Zwaan M, Herpertz S, Zipfel S, Svaldi J, Friederich H-C, Schmidt F, et al. Effect of internet-based guided self-help vs individual face-to-face treatment on full or subsyndromal binge eating disorder in overweight or obese patients: the INTERBED randomized clinical trial. JAMA Psychiatry (2017) 74:987–95. doi: 10.1001/jamapsychiatry.2017.2150
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Bulik CM, Marcus MD, Zerwas S, Levine MD, Hofmeier S, Trace SE, et al. CBT4BN versus CBTF2F: comparison of online versus face-to-face treatment for bulimia nervosa. Contemp Clin Trials (2012) 33:1056–64. doi: 10.1159/000449025
PubMed Abstract | CrossRef Full Text | Google Scholar
70. Fairburn CG, Bailey-Straebler S, Basden S, Doll HA, Jones R, Murphy R, et al. A transdiagnostic comparison of enhanced cognitive behaviour therapy (CBT-E) and interpersonal psychotherapy in the treatment of eating disorders. Behav Res Ther (2015) 70:64–71. doi: 10.1016/j.brat.2015.04.010
PubMed Abstract | CrossRef Full Text | Google Scholar
71. Qi H, Zhu F, Qi MH. Package ‘SampleSize4ClinicalTrials’. (2022).
Google Scholar
72. Chow S, Shao J, Wang H. Sample Size Calculations in Clinical Research. 2nd Ed. Chapman & Hall/CRC Biostatistics Series. (2008).
Google Scholar
73. Yin G. Clinical Trial Design: Bayesian and Frequentist Adaptive Methods. John Wiley & Sons. (2012).
Google Scholar
74. Cohen LJ. The Probable and the Provable Oxford: Clarendon Press UK (1977) Vol. 384. doi: 10.1093/acprof:oso/9780198244127.001.0001
CrossRef Full Text | Google Scholar
75. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Statistics (1981) 6:107–28. doi: 10.3102/10769986006002107
CrossRef Full Text | Google Scholar
76. Zwaap J, Knies S, van der Meijden C, Staal P, van der Heiden L. Cost-effectiveness in practice Diemen, the Netherlands: Zorginstituut Nederland (2015).
Google Scholar
78. IBM Corp. IBM SPSS Statistics for Windows, Version 28.0, Armonk, New York, USA. (2021).
Google Scholar
79. Custers B, Sears AM, Dechesne F, Georgieva I, Tani T, van der Hof S. EU personal data protection in policy and practice. Dordrecht, the Netherlands: Springer (2019).
Google Scholar
80. Cooper Z, Allen E, Bailey-Straebler S, Basden S, Murphy R, O’Connor ME, et al. Predictors and moderators of response to enhanced cognitive behaviour therapy and interpersonal psychotherapy for the treatment of eating disorders. Behav Res Ther (2016) 84:9–13. doi: 10.1016/j.brat.2016.07.002
PubMed Abstract | CrossRef Full Text | Google Scholar
81. Stuhldreher N, Konnopka A, Wild B, Herzog W, Zipfel S, Löwe B, et al. Cost-of-illness studies and cost-effectiveness analyses in eating disorders: a systematic review. Int J Eat Disord (2012) 45:476–91. doi: 10.1002/eat.20977
PubMed Abstract | CrossRef Full Text | Google Scholar
82. Preschl B, Maercker A, Wagner B. The working alliance in a randomized controlled trial comparing online with face-to-face cognitive-behavioral therapy for depression. BMC Psychiatry (2011) 11:189. doi: 10.1186/1471-244X-11-189
PubMed Abstract | CrossRef Full Text | Google Scholar
83. Hilbert A, Petroff D, Herpertz S, Pietrowsky R, Tuschen-Caffier B, Vocks S, et al. Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J Consult Clin Psychol (2019) 87:91–105. doi: 10.1037/ccp0000358
PubMed Abstract | CrossRef Full Text | Google Scholar
84. Blom TJ, Mingione CJ, Guerdjikova AI, Keck PE, Welge JA, McElroy SL. Placebo response in binge eating disorder: a pooled analysis of 10 clinical trials from one research group. Eur Eat Disord Rev (2014) 22:140–6. doi: 10.1002/erv.2277
PubMed Abstract | CrossRef Full Text | Google Scholar