Participants
Twenty-three women with binge eating were recruited for the study. One participant withdrew from the study due to vomiting during the test day. Unblinding revealed the participant had received LDX on this test day. The sample size was based on the results of a previous study that assessed the effects of a 5-HT2C receptor agonist on food intake using similar paradigms (effect size of 0.67) [12]. A power analysis (G*power 3.1.9.7) [22] indicated a sample size of 20 participants was needed to obtain 80% power to detect such an effect at alpha = 0.05. To allow for smaller effect size and for dropouts, we initially aimed to recruit 35 participants. However, due to the global pandemic caused by the novel coronavirus SARS-CoV-2, all in-person data collection was halted, and the resulting sample size was 22 (M age = 24.41 ± 6.87, M BMI = 26.35 ± 4.98).
Participants were invited to take part if they met the eligibility criteria (See Supplementary Table 1) and were recruited via posters and social media platforms. The study was approved by the National Research Ethics Service and was pre-registered on clinicaltrials.gov as NCT04181957.
Design
In a double-blind, placebo-controlled, crossover design, participants meeting inclusion criteria, and having given full informed consent, were randomised prior to the test day by a researcher not involved in data collection to receive oral LDX (50 mg) in a single morning dose, or placebo, in a counterbalanced order. The LDX and placebo were prepared by Guy’s and St Thomas’ NHS Foundation Trust Pharmacy. Both LDX and placebo were prepared in identical capsules to maintain blinding. Previous research indicates that 50 mg LDX is a clinically effective dose with few side effects [3, 4]. All participants took part in two sessions on two separate days, at least 7 days apart.
Eating-related measures
Food was served on the Sussex Ingestion Pattern Monitor (SIPM), which consists of a balance placed underneath the surface of a table covered by a placemat [12]. The balance was connected to a laptop that recorded the weight of the plate and alerted the participant each time 50 mg of pasta was consumed, or 10 g of cookies was consumed, at which point the participant was instructed to complete VAS ratings of hunger, fullness, and pleasantness of the meal. Eating rate was calculated as grams eaten/total time spent eating (minutes). Lunch comprised pasta shells in a tomato and herb sauce (both Sainsbury’s brand) served at 55–60 °C (233 kilocalories per 200 g) and ad libitum water. After 150 g had been consumed, the participants were interrupted, and the plate was replaced with a fresh 200 g plate of pasta. Participants were instructed to continue to eat as many plates as they wished until they were comfortably full. Maryland brand chocolate chip cookies were offered ad libitum 15 min after the pasta meal. Participants were served a bowl containing 80 g (approximately 396 kilocalories) of cookies broken into bite-size amounts to avoid participant tracking of amount consumed. When 60 g of cookies were consumed, participants were provided with a fresh bowl containing 80 g and could continue in this manner until they wished to stop.
Cognitive Tasks
P1vital® Oxford emotional test battery (ETB)
The ETB is a computerised battery that comprises validated cognitive tasks to determine emotional bias [20].
Emotional categorisation (ECAT): Sixty positive and negative adjectives (e.g., cheerful, hostile) were presented in white text on a black screen. Each adjective was presented for 500 ms. The participant was instructed to select if they would ‘like’ or ‘dislike’ to be described as such as quickly and accurately as possible. Accuracy and reaction time (RT) by valence are reported.
Emotional recall (EREC): The participants were asked to recall as many words from the ECAT as could be remembered within a 4 min period. Participants wrote their responses on paper. The number of correct words recalled by valence and commission errors are reported.
Emotional recognition memory (EMEM): Participants were presented with the 60 words from the ECAT, along with 60 matching novel distractor words, on a black screen. The participants were instructed to indicate whether the word had been presented during the ECAT trial. Accuracy, RT, and commission errors by valence are reported.
Facial expression recognition (FERT): Faces with one of six emotional expressions (happiness, fear, anger, disgust, sadness and surprise) or a neutral expression appeared on a black background screen. The faces were morphed from neutral to full expressions in 10% increments to foster ambiguity about the expression being displayed. Each intensity was represented four times, along with ten presentations of neutral expressions totalling 250 stimuli. Each stimulus was presented for 500 ms, followed by a blank screen. The participant was instructed to classify each expression as quickly and as accurately as possible. Accuracy, commission errors, and RT by valence are reported.
Stop-signal task (SST)
The SST is a measure of response inhibition [23]. This task was adapted from the STOP-IT software programmed by Verbruggen et al. [23]. A white arrow was presented on a black background, pointing either left or right. The participant indicated the direction of the arrow using the left and right keys on the keyboard. On a subset of these trials (‘stop trials’), the white arrow turned blue in colour, indicating that the participant had to attempt to inhibit their motor response on the given trial (as instructed in advance of doing the paradigm). The blue arrow in stop-signal trials is initially presented for 250 ms, and this delay is then adjusted using the staircase tracking procedure whereby the personalised adjusted score is the stop-signal delay (SSD). The experiment consists of three blocks of 64 trials in which 75% of the trials are no-signal trials. The stop-signal reaction times (SSRT) indicates the time taken by the individual to suppress a response that would normally be made and is calculated by subtracting mean SSD from mean RT. Omission and commission errors, RT for no-signal and stop-signal trials (SSRT), and SSD are reported.
N-back
The participant was presented with a sequence of blue circles on a white 3 × 3 grid and was instructed to indicate whether the current circle location matched or did not match the location of the circle 2 (2-back) or 3 (3-back) trials earlier. Participants completed 70 trials of each condition with a break between the 2 and 3-back. Accuracy and RT for each condition (2 back or 3 back) are reported.
Continuous performance test
A series of white letters were presented on a grey background in a random order [24, 25]. Participants were instructed to press the space bar for every letter except ‘X’. Letters were presented for 900 ms. The ‘X’ appeared in 42 of the 830 trials. An average of the RT standard deviations (SDRT) was calculated to measure response time variability (RTV). Increased RTV is considered to reflect poorer ability to sustain attention [26]. Commission errors provide a measure of impulsive responding, while omission errors provide a measure of inattention [27]. Omission and commission errors, RT and SDRT/RTV are reported.
fMRI picture rating task
During fMRI, participants performed a picture rating task [12]. Participants viewed food and non-food stimuli (36 from each category and visually matched). The food pictures varied in fat and sugar content (high fat, high sugar; high fat, low sugar; low fat, high sugar and low fat, low sugar). Items were rated on how appealing they were on a scale from 1 (not at all) to 5 (very much) using a button box. Each picture was presented for 1500 ms, followed by a fixation cross (500–1500 ms).
Acquisition, processing and analysis of fMRI data
Imaging data were collected using a Siemens MAGNETOM Prisma 3 T MRI system at the Centre for Human Brain Health (CHBH), University of Birmingham. Functional images during the picture rating task (3× 300 volumes) were acquired with single-shot echo-planar imaging (EPI) sequence as described in the supplementary materials. Data were analysed using SPM12 (Wellcome Department of Imaging Neuroscience, London, UK) run with MATLAB 2019 (Mathworks Inc, Natick, MA) using standard procedures [28] (supplementary materials).
Procedure
Participants attended a screening day and a separate test day. On the screening day, binge eating was confirmed using the Binge-Eating Scale (BES) [29]. Participants were eligible to take part if they had a Moderate score (18–26) or Severe score (27–46). The Structured Clinical Interview for DSM-5, Clinical Version (SCID-CV) [30] was also completed on the screening day to exclude participants with other mental health conditions, including Anorexia Nervosa and Bulimia Nervosa. On the test day, participants arrived at 8:30 or 9:00 after eating their usual breakfast (to standardise hunger). After the LDX or placebo capsule was self-administered, participants waited for 2 h for peak drug levels to be achieved and during this time they completed the Dutch Eating Behaviour Questionnaire (DEBQ) [31], and the Barratt Impulsiveness Scale (BIS-11) [32]. From 11:00 am they completed the following tasks in order: ETB, SST, n-back, fMRI session. The fMRI session started around 12:30. During the fMRI scan, participants completed a delay discounting task (data not reported here) and the picture rating task. Following the scan, lunch was offered and then participants completed the inattention task before consuming the cookie snack. Throughout the day, VAS assessing mood and physical state were completed. A total of 14 items were rated using a 0 cm (‘completely absent’) to 10 cm (‘most I could imagine’) scale: alertness, drowsiness, happiness, hunger, fullness, desire to eat, disgust, anxiety, sadness, withdrawn, lightheaded, nausea and faint. A total of 3 mL blood samples were collected via venipuncture for assessment of d-amphetamine concentration (mg/L) by Analytical Services International Ltd. The results confirmed no presence of d-amphetamine in baseline blood samples and expected plasma levels of D-amphetamine after 275 min (0.05 mg/L) and 325 min (0.06 mg/L) post-dosing (see Fig. 1 for a summary of the test day).
Fig. 1: Timeline of screening and testing.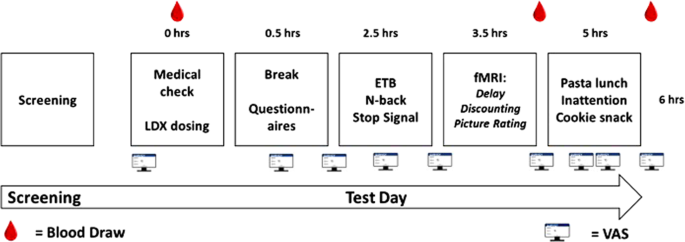
Flow diagram showing an overview of the screening and test days timings in hours (hrs).
Data and statistical analysis
Performance-based exclusion criteria were determined prior to data analysis. Outlying data points, which were defined as below 200 ms and ≥ 6000 ms for RTs and outside 3*interquartile range of the lower and upper grand mean for other performance measures were removed. In addition, participants scoring at below chance performance (less than 50%) on the EMEM and N-back tasks were removed from the analysis, which resulted in smaller degrees of freedom for these tasks. Using the factor structure calculated by Thomas et al. [15], VAS factors consisted of ‘Arousal’ (alertness, drowsiness, and happiness), ‘Appetite’ (hunger, fullness, and desire to eat), ‘Negative Effects’ (disgust, anxiety, sadness, and withdrawn), ‘Physical Effects’ (lightheaded, nausea, and faint), and thirst [15]. VAS factors were converted to AUC using the trapezoid method. Regression imputations were used to replace missing VAS data. The data met the assumptions for parametric testing. Unless noted otherwise, the data were analysed using repeated-measures ANOVA. Main effects and interactions that did not involve drug conditions are not reported and comparisons are reported for LDX versus placebo.

