Trauma, PTSD, and eating disorders
Scientific evidence for the association between trauma, PTSD and other trauma-related disorders in the predisposition, precipitation and perpetuation of eating disorders (EDs), has been established in a variety of samples, including treatment and non-treatment seeking individuals of various ages, genders and sexual orientations (1–6). The pooled lifetime prevalence rates of PTSD in EDs (ED + PTSD) in the highest quality studies average 25% with higher rates of 37–45% in bulimia nervosa (BN) and 21–26% in binge eating disorder (BED) (7–9). Lower prevalence rates are reported in association with AN, particularly the restricting type (10–14%). Nevertheless, higher rates of PTSD are reported in all ED patients admitted to residential care, especially those with BN, anorexia nervosa binge-purge type (AN-BP), and other specified feeding and eating disorders (OSFED) (2, 10, 11). Conversely, higher rates of EDs are also seen in patients with PTSD compared to the general population (8, 12).
It is common for ED + PTSD patients to report histories of multiple traumas and/or trauma types (1, 2, 10, 13). High trauma “doses” have been associated with ED severity and comorbidities (2, 13–16), and trauma histories and PTSD symptoms have been reported to predict more complicated courses of illness, higher dropout rates, and worse outcomes following treatment (11, 17–26). Evidence also suggests that individuals with ED + PTSD may be significantly more impulsive, prone to revictimization, and the subsequent perpetuation of PTSD (27–30), which apart from EDs tends to be a chronic disorder (31–33). Taken together, the development and adoption of integrated treatment approaches for ED + PTSD is warranted.
Existing ED guidelines lack a trauma-PTSD focus
Existing guidelines for the treatment of EDs do not adequately address the problem of psychiatric comorbidity, which is more common than not in this group of disorders. Although admittedly this is a complicated topic, previously published ED guidelines often mention the entire issue of psychiatric comorbidity in a rather cursory manner and, if mentioned at all, do not address the depth of discussion or nuance that is often necessary to address ED + PTSD. In the excellent 2017 review of 8 existing guidelines for AN by Hilbert and colleagues, only 3 of these 8 manuscripts mentioned psychiatric comorbidities, while 5 of 9 guidelines for BN noted comorbidities, usually as “special issues” (34).
The issue of trauma and specifically of PTSD in the assessment of EDs was noted in the Australian and New Zealand guideline. These experts noted that psychiatric comorbidity occurs as high as 55% in community adolescent samples and up to 96% in adult samples, and they state, “Comorbidity in people with anorexia nervosa is common and therefore assessment for such should be routine” (35). They also make the recommendation to “be prepared to treat comorbidity to improve quality of life,” although no specifics as to how to do this are offered other than using CBT-E, which does not directly address trauma or PTSD, and considering adjunctive pharmacotherapy (35).
The updated German guidelines for the treatment of AN specifically noted, “many patients are affected by comorbid psychological diseases,” and that comorbid conditions, such as PTSD, “might require changes in treatment planning and prioritization of therapy goals,” although no specifics were provided (36).
More recently the American Psychiatric Association published an updated practice guideline for the treatment of patients with eating disorders, which notes that “eating disorders frequently co-occur with other psychiatric disorders” (37, 38). The guideline states that “all patients with a possible eating disorder should be asked about a history of trauma … and assessed for symptoms related to PTSD.” Although “data are…limited on individuals with eating disorders and…co-occurring psychiatric disorders,” the guideline recommends that psychotherapy in adults with AN “address comorbid psychopathology, psychological conflicts, adaptive benefits of symptoms, and family or cultural factors that reinforce or maintain eating disorder behaviors,” suggestions which are certainly applicable to the treatment of ED + PTSD in patients with any type of ED.
Notably, none of the guidelines for adult patients with EDs that were reviewed for this commentary specifically addressed treatment of ED + PTSD per se. However, in the newly published Australian guideline for the treatment of ED patients at higher weights, much more attention to trauma, PTSD, and trauma-informed care is paid (39). This is relevant given that ED patients with PTSD treated in higher levels of care have been reported to have significantly higher BMIs (2, 10, 40). Importantly, Ralph and colleagues offer lived experience perspectives, and specifically discuss trauma-informed care within an ED context for patients in larger bodies. They also acknowledge how ED treatment itself may be traumatizing and how PTSD and other comorbidities occur “frequently” (39).
Three published guidelines were found for the treatment of children and adolescents with EDs (41–43). The focus on psychiatric comorbidity was most prominent in the guideline from the American Academy of Child and Adolescent Psychiatry, which states “further complicating the diagnosis of AN is the potential presence of other significant psychiatric comorbid conditions” (43). Abuse is noted to be a potential risk factor for BN, and PTSD an associated comorbid disorder in BN and BED, but no specific treatment guidelines for ED + PTSD are provided other than to recommend that “guidelines for the specific condition should be followed” and “The use of medications, including complementary and alternative medications, should be reserved for comorbid conditions and refractory cases.” The Canadian guideline for children and adolescents with EDs only mentions trauma and PTSD in reference to ARFID (41). However, it notes in the very last line of the guidelines the following statement: “Research efforts should be devoted to developing treatments for severe eating disorders with complex comorbidity.”
Existing PTSD guidelines lack an eating disorder focus
Conversely, upon reviewing available practice guidelines for the assessment and treatment of PTSD or complex PTSD, the treatment of EDs is never addressed (44–54). The only PTSD guidelines that specifically mention EDs as an associated comorbidity are those of the American Psychiatric Association (55). Several guidelines specifically note that the presence of comorbid disorders should not prevent patients from receiving trauma-focused treatments (51, 52, 54, 56). However, in my experience trauma specialists are often unprepared to deal with PTSD patients with active ED symptoms. Unfortunately, it has been a common but unfortunate occurrence for ED + PTSD patients to be refused treatment in either an ED specialty program or trauma specialty program, or if they are admitted, only the so-called “primary” disorder (for insurance purposes) is treated. The treatment of one disorder to the exclusion of the other is often inadequate in that it all too often results in poor outcomes.
Toward the development of treatment guidelines for ED + PTSD and related comorbidity
Recommendations regarding the treatment of ED + PTSD and related comorbidity have been previously outlined and discussed (1, 57, 58), and an updated, expanded conceptualization of these guidelines and principles is discussed here. Possible recovery pathways that may facilitate integrated treatment planning are shown in Figure 1. In the figure, the process starts on the left and proceeds in time to the right from Assessment (Timeline) to Diagnosis to Treatment (Tx) to Response as the patient ideally proceeds from highly symptomatic on the left to maintenance and/or remission on the far right, followed by relapse prevention and/or recurrence and reassessment.
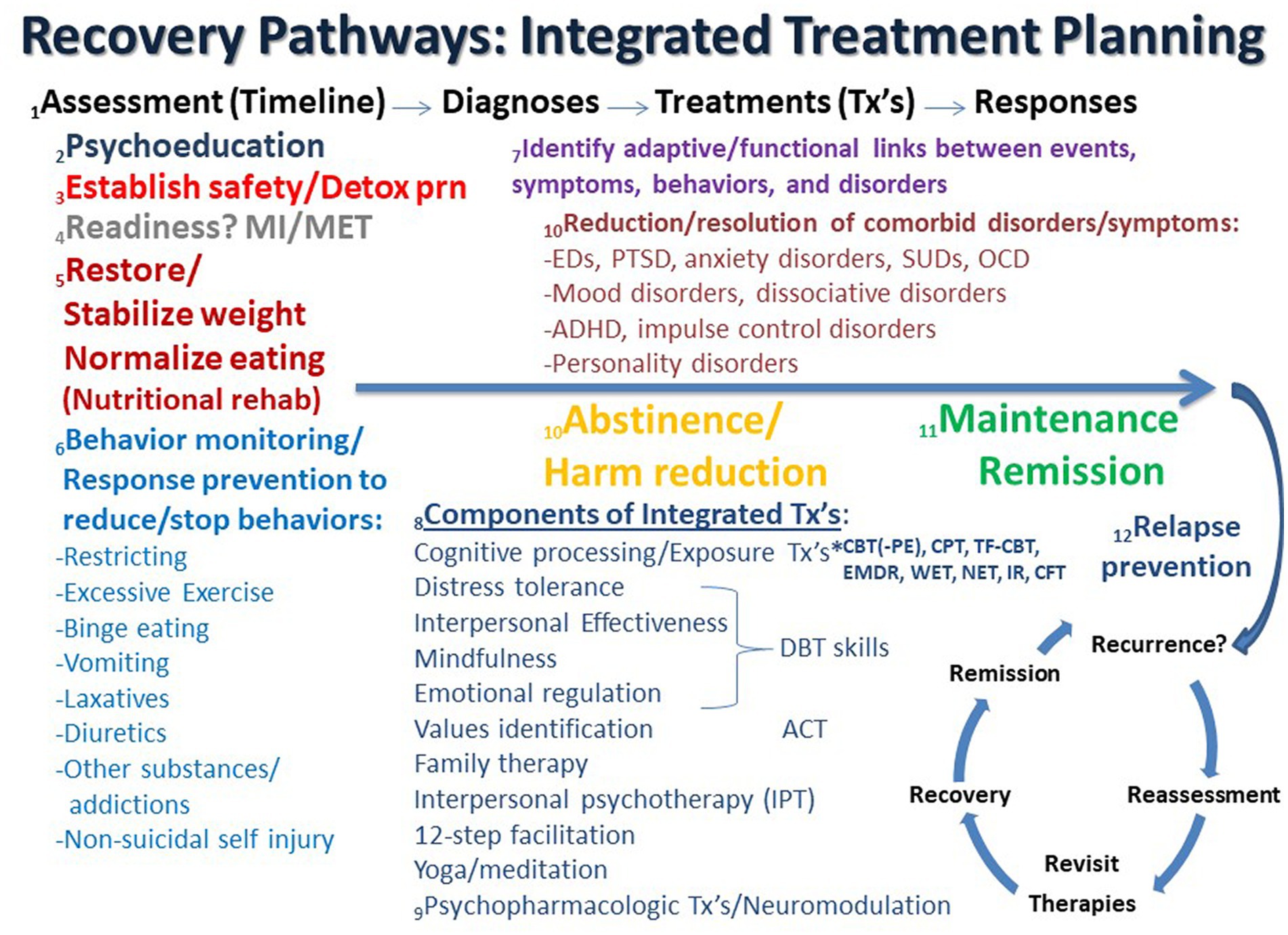
Figure 1. Recovery pathways that facilitate integrated treatment planning are discussed consecutively in the text as numbered. ACT, acceptance commitment therapy; ADHD, attention deficit hyperactivity disorder; CBT, cognitive behavioral therapy; CFT, compassion focused therapy; CPT, cognitive processing therapy; Detox, detoxification; DBT, dialectical behavior therapy; EMDR, eye movement desensitization reprocessing; IR, imaging rescripting; IPT, interpersonal psychotherapy; MI, motivational interviewing; MET, motivational enhancement therapy; NET, narrative exposure therapy; OCD, obsessive compulsive disorder; PTSD, posttraumatic stress disorder; PE, prolonged exposure; SUD, substance use disorder; TF-CBT, trauma-focused cognitive behavioral therapy; Tx’s, treatments; WET, written exposure therapy.
1. Assessment (Timeline) – Diagnosis: Most guidelines recognize the importance of “a comprehensive assessment of the individual and their circumstances” (35). It has been previously noted that “the most tried and true initial approach for any patient with or without complex symptomatology is to perform a complete psychiatric evaluation, which substantially reduces the chances of misdiagnosis” (57). This remains as important as ever, especially in an era in which time-limited evaluations are the norm in most treatment settings. Therefore, assessment is often an ongoing process that unfolds over several sessions. As part of this complete medical and psychiatric evaluation, the clinician and patient collaboratively develop a timeline that depicts the chronology of significant life events, traumas, symptom/disorder onsets, remissions, and relapses in the individual’s lifetime. Such a timeline can form the basis of the patient’s life narrative, including history of traumatic events and important contexts, and allows the patient and clinician to recognize and discuss predisposing, precipitating, perpetuating, and palliative factors in the patient’s life. Such an approach is compatible with the emerging paradigm of a life course approach as well as the concept of personalized medicine to address multimorbidity (59–63). It is important to note that the development of the patient’s historical timeline may occur over an extended period of time as therapy progresses and evolves (see #1 in Figure 1). Many ED patients do not feel comfortable revealing traumatic events during the initial assessment but need time to develop a trusting alliance with the therapist before such history is disclosed. The formation and maintenance of a therapeutic alliance remains a cornerstone of future work and progress (64, 65). Therapists need to convey a sense of safety, trust, honesty, straightforwardness, compassion, knowledge, and nonjudgmental positive regard, which are essential elements for any successful treatment course. Countertransference also needs to be closely monitored and contained (66–68). Cognitive processing therapy (CPT) emphasizes the importance of therapists avoiding avoidance of addressing traumatic material (69), as avoidance is highly characteristic of both EDs and PTSD (6, 11, 70–75). It has been previously noted that it is not uncommon for patients with ED + PTSD to not even realize that they have been victimized until the definitions of trauma and abuse types are explained to them (57). This is particularly relevant for patients with child maltreatment and complex PTSD. Only later after a substantial degree of cognitive development has occurred do some individuals realize the extent and meaning of their traumas.
2. Psychoeducation: As a comprehensive list of problems and conditions is being determined, the clinician moves forward educating the patient and supportive family about all diagnosed disorders, both current and lifetime. Psychoeducation is the cornerstone of all modern therapies, especially cognitive-behavioral and trauma-focused approaches (see #2 in Figure 1). Psychiatric disorders often vacillate in and out of remission, and previous disorders may serve as important predisposing factors for subsequent symptoms and disorders. Notably, comorbidity is the rule rather than the exception in PTSD and EDs (57, 76–78), and comorbid disorders are generally not contraindications to trauma-focused treatments (79, 80). Such treatments can be safely and effectively used in patients with a range of psychiatric disorders and are often associated with concurrent decreases in PTSD and the comorbid problem(s) (80–83).
3. Safety and stability: The most important determination and goal early on in treatment is to initially assess and address the greatest danger or risk to life, e.g., suicidality, self-harming behaviors, starvation, fluid/electrolyte disturbances, cardiac arrhythmia, etc. (see #3 in Figure 1). Attaining relative safety and stability is the foundation of stage 1 treatment of PTSD with complex presentations (complex PTSD), as well as dissociative disorders, which are highly linked to developmental traumas (46, 84–87). Given its pervasive association with problems in self-regulation, ED + PTSD with related comorbidities may be conceptualized as forms of complex PTSD (1, 88–90). Emerging behaviors that derail treatment progress, or therapy interfering behaviors (91–93), may need to be addressed as the therapist assists the patient to identify avoidance and work on stuck-points, cognitive distortions, or maladaptive beliefs (69). However, some trauma experts question the need for a phasic approach, arguing that there is a lack of research to support several contentions, including that: (1) a phase-based approach is necessary to attain positive outcomes, (2) trauma-focused treatments have unacceptable risks, and (3) response to trauma-focused treatments are improved when preceded by a stabilization phase (94). On the other hand, ISTSS guidelines indicate sufficient evidence and clinical rationale to support the phase-oriented approach with an initial period of stabilization and establishment of safety (46, 84, 95). Suicidality and other immediately life-threatening medical conditions are effectively the major primary contraindications against initiating trauma-focused treatment (79). When a substance use disorder (SUD) complicates the clinical picture, a detox strategy that is appropriate for the particular substance is an important early treatment component that is subsumed under the goal of establishing safety and stability.
4. Readiness for change: As assessment and treatment move forward, it is incumbent on therapists to identify readiness for change and willingness to engage in both PTSD and ED recovery, which are intimately interwoven (96–100). Motivational interviewing (MI) and motivation enhancement therapy (MET) approaches may help to identify the most problematic condition per the patient’s perspective and can be important prequels and/or adjuncts to other subsequent evidence-based therapies for EDs, PTSD and related comorbidities (101–108) (see #4 in Figure 1). However, it is notable that readiness for trauma-focused treatment is not always accurately assessed by therapists, which can lead to inadvertent collusion with avoidance (101).
5. Nutritional rehabilitation: Early on in treatment it is essential to establish that the patient has begun the process of nutritional rehabilitation, is more adherent to this plan than not (progress not perfection), is medically stable, and can begin to process information emotionally and cognitively (see #5 in Figure 1). New learning is dependent upon adequate food intake and subsequent protein and neurotransmitter synthesis (109–111). Ideally, effective psychotherapy requires grossly intact brain function, the ability to attend to the process at hand, and the ability and motivation to learn new information (and unlearn maladaptive strategies). Starved, intoxicated and/or severely dysregulated brains are unable to learn well and therefore are less likely to benefit from psychotherapy. Nevertheless, trauma-focused treatment should not be delayed until full weight restoration or remission of ED behaviors is achieved as long as the patient is safe and willing to engage in trauma processing. Evidence has been accumulating that the long-term resolution of ED symptoms may in fact hinge on trauma-focused treatment (4, 77, 112, 113).
6. Behavior monitoring and response prevention: Just as psychoeducation is an integral early component of cognitive behavioral therapies, so too is behavior monitoring and response prevention of problematic behaviors. Patients are asked to record instances and frequencies of targeted behaviors, including food intake and episodes of binge eating, purging, excessive exercise, substance use, and non-suicidal self-injury. This prescription is not only a part of ongoing assessment but is an effective treatment intervention in itself (see #6 in Figure 1). As treatment continues, the therapist periodically confirms that ED behaviors are being sufficiently addressed in an ongoing manner. Monitoring of ED and PTSD symptoms using validated measures, e.g., the Eating Disorder Examination Questionnaire (EDEQ) (114) and the PTSD Symptom Checklist for DSM-5 (PCL-5) (115), respectively, are essential in assessing progress for the ED + PTSD patient, while an array of other assessments are available for other comorbidities. While full remission may remain elusive, it is helpful to frame various degrees of symptom improvement as evidence of progress and success. Combating perfectionistic, “all or nothing” thinking is often an ongoing process in the long-term treatment of ED + PTSD patients, and teaching and promoting the concept and goal of harm reduction is a useful endeavor that has application to multiple symptoms and disorders on the comorbidity spectrum (116–118).
7. Adaptive function: An important part of the therapeutic process is to collaboratively identify the relationships between life events, symptoms of PTSD, eating and other disorders, which may serve as adaptive functions, such as to facilitate avoidance and numbing, decrease hyperarousal, regulate trauma-related states (“self-medication hypothesis”) (119) or solve some perceived problem(s) (15). Certain ED behaviors may also be traumatic reenactments (120). “Building the bridge” between trauma-related/PTSD symptoms and ED behaviors/symptoms serves to foster new connections and meanings and paves the way toward sustained recovery (see #7 in Figure 1). Continued work on the patient’s timeline can facilitate ascertaining these connections. Establishing the interconnectedness of and bridging between symptoms is supported by recent network analyses of ED and PTSD symptoms (97, 99, 100).
8. Components of integrated treatment: psychotherapies: It is argued that an integrated trauma-focused treatment plan using a rational mixture of evidence-based techniques and tools is indicated. An overview of the psychotherapeutic components that may be considered in integrated treatment planning is shown in Figure 1 (see #8). The field has evolved beyond the “one size fits all” approach, which is woefully inadequate for the ED + PTSD patient. Generally, some form of trauma-focused treatment is indicated for PTSD, and this should be no different when there is ED comorbidity. The most researched of trauma-focuses approaches in this population is cognitive processing therapy (CPT), which arguably is very well-suited for ED + PTSD patients (29, 112, 113, 121–123). However, other trauma-focused therapies can be applied to ED + PTSD patients, including prolonged exposure (PE), eye movement desensitization reprocessing (EMDR), imaging rescripting (IR), written exposure therapy (WET), narrative exposure therapy (NET), compassion-focused therapy (CFT), and trauma-focused CBT (TFCBT) in children and adolescents (49, 71, 121, 124–142). Other adjunctive approaches, or elements of these approaches, can also be useful, including acceptance and commitment therapy (ACT), which identifies values and addresses experiential avoidance (143–146), interpersonal psychotherapy (IPT), which has been reported to be effective in EDs, PTSD, and major depression (145, 147–152), and 12-step facilitation, a manual based, therapist driven treatment based on 12-step principles found to be effective for alcohol use and stimulant use disorders (153–155). The unified treatment model has also been recommended for ED + PTSD (156). Self-administered emotional freedom techniques (EFT) have been successfully employed for PTSD, but have not been systematically explored in EDs (157). Family therapy with non-offending parents and/or guardians is an essential ingredient for children and adolescents with ED and PTSD, and it should be considered for all patients of any age (158, 159). Involving family members in assessment and treatment is often extremely helpful and can contribute to significant therapeutic advances. When patients are not amenable to involving family members it is important for therapists to understand why.
A sufficient level of distress tolerance and emotional regulation is required so that psychotherapy can proceed, although pauses and delays are likely to occur in the course of addressing patients with complex psychopathology. The use of DBT skills as grounding techniques to enhance self-regulation, mindfulness, distress tolerance and interpersonal effectiveness can be essential, powerful adjuncts to trauma-focused therapies for severely ill comorbid ED + PTSD patients (160, 161). Other grounding or anxiety reduction techniques, such as focused breathing, yoga and other meditation practices, can be utilized as evidence-based treatment adjuncts (162–172). However, a certain amount of emotionality is to be expected and should not in itself be a reason for delay. A central tenet of evidence-based trauma-focused treatment is for the therapist to “avoid avoidance” and to not collude with the patient’s tendency to deflect (69). Disengagement coping has been found to predict revictimization, while engagement coping predicts better outcomes (173).
9. Components of integrated treatment: psychopharmacology and neuromodulation: Psychopharmacological interventions are also effective evidence-based treatments for non-comorbid EDs, PTSD and related comorbid disorders, e.g., mood and anxiety disorders, but they should always be adjunctive to psychotherapeutic interventions for patients with ED + PTSD and related comorbidities (174–179) (see #9 in Figure 1). The agents that have been found to be beneficial in ED and PTSD samples separately generally include antidepressants, especially fluoxetine and other serotonin reuptake inhibitors, atypical antipsychotics, especially olanzapine, and anticonvulsants, especially topiramate (35, 180–199). In addition, naltrexone can be highly effective in reducing alcohol intake as well as combating non-suicidal self-injury and binge eating (200–210). Other novel approaches to consider, especially when treatment-refractory major depression is a focus, may include the use of newer psychopharmacologic agents, e.g., 5-HT4 receptor antagonists (211), combining other previously unused evidence-based psychotherapies and psychopharmacologic approaches (142, 212), adjunctive application of neuromodulation tools, such as repetitive transcranial magnetic stimulation (rTMS) (213, 214), deep TMS (215) or electroconvulsive therapy (ECT) (216), novel psychotropic-or psychedelic-assisted therapies, such as ketamine/esketamine, 3,4-methylenedioxymethamphetamine (MDMA), psilocybin, and ayahuasca (12, 28, 212, 217–231), as well as deep brain stimulation (DBS) (232–235), although many of these newer approaches continue to be preliminary, experimental and/or challenging to acquire.
10. Resolution, abstinence, and harm reduction: It is well known that improvement in one set of symptoms often results in the resolution of or improvement in related symptoms of other disorders, which exist in a network with each other (see #10 in Figure 1) (147, 236–238). For example, as ED symptoms abate with treatment so also can symptoms of disorders of mood, anxiety, personality, etc. (11, 112, 122, 239, 240). Similarly, abstinence from or a reduction in SUD behaviors often accompanies similar reductions in mood, anxiety and other comorbid symptoms (241, 242). Likewise, recovery from PTSD often results in improvements in a host of related psychological symptoms (243–245).
11. Maintenance, remission, and relapse prevention: As improvements continue and degrees of remission are achieved, patients may move into the maintenance phase of treatment (see #11 in Figure 1). It is during this phase that relapse prevention strategies can be successfully employed. Specific forms of CBT and pharmacotherapy focused on relapse prevention have been found to significantly reduce relapse for eating, substance use, mood and anxiety disorders (246–255).
12. Refractoriness and relapse: In the face of refractoriness to treatment, inadequate response, and/or relapse, which is common in EDs and related comorbidities (256–258), it is helpful for the patient and therapist to re-evaluate the treatment plan, identify triggers, stuck points, and potential weak points, and to revisit therapeutic options (see #12 in Figure 1). Experienced clinicians are familiar with the “whack-a-mole” phenomenon in which one or more problems resolve as others emerge. When the therapeutic skills of the therapist do not match the needs of the patient, then it is sometimes appropriate and in the best interests of the patient to discuss switching gears and referring to someone else with greater expertise, experience or skills. However, it is essential that hope continue to live eternal while the odds of attaining desired goals are evaluated. The lived experiences of patients with ED + PTSD, particularly those with severe and enduring eating disorders, should be carefully considered in light of what is and is not known about long-term outcome data (259–270). Despite the availability in some locations of euthanasia and physician-assisted suicide for so-called “terminal anorexia,” it is my and others’ opinion that this course of action not be considered a possible option (263, 271–273), while palliative care may be indicated and considered (274). There are so many potential therapeutic options and combinations of options that one is hard pressed to argue that all avenues have been exhausted in the face of ED + PTSD chronicity and refractoriness.
In conclusion, effective treatment of ED + PTSD and related comorbidity requires the therapist to acquire many needed skills and resources, many of which are outlined in Figure 1 and discussed in this commentary. Abraham Maslow once said, “If all you have is a nail, then everything is a hammer.” Applying only one therapeutic approach to every ED patient is insufficient, a conclusion that is hopefully obvious at this juncture. Available clinical research evidence suggests that continuing with traditional single-disorder focused, sequential treatment models with multiple providers that do not prioritize integrated, trauma-focused treatment approaches are short-sighted and often inadvertently perpetuate this dangerous multimorbidity. Future ED practice guidelines would do well to address concurrent illness in more depth. Taken together, these principles offer the clinician a map for better negotiating a successful journey of recovery for the ED + PTSD comorbid patient. Recently published outcome data indicate that integrated treatment approaches can result in significant improvement in ED, PTSD and related symptoms (11, 12, 112, 122, 127, 156).
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
TB is the owner of Timothy D. Brewerton, MD, LLC.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brewerton, TD. An overview of trauma-informed care and practice for eating disorders. J Aggress Maltreat Trauma. (2018) 28:445–62. doi: 10.1080/10926771.2018.1532940
CrossRef Full Text | Google Scholar
2. Brewerton, TD, Perlman, MM, Gavidia, I, Suro, G, Genet, J, and Bunnell, DW. The association of traumatic events and posttraumatic stress disorder with greater eating disorder and comorbid symptom severity in residential eating disorder treatment centers. Int J Eat Disord. (2020) 53:2061–6. doi: 10.1002/eat.23401
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Brewerton, TD, Suro, G, Gavidia, I, and Perlman, MM. Sexual and gender minority individuals report higher rates of lifetime traumas and current PTSD than cisgender heterosexual individuals admitted to residential eating disorder treatment. Eat Weight Disord. (2022) 27:813–20. doi: 10.1007/s40519-021-01222-4
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Mitchell, KS, Scioli, ER, Galovski, T, Belfer, PL, and Cooper, Z. Posttraumatic stress disorder and eating disorders: maintaining mechanisms and treatment targets. Eat Disord. (2021) 29:292–306. doi: 10.1080/10640266.2020.1869369
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Reyes-Rodriguez, ML, Von Holle, A, Ulman, TF, Thornton, LM, Klump, KL, Brandt, H, et al. Posttraumatic stress disorder in anorexia nervosa. Psychosom Med. (2011) 73:491–7. doi: 10.1097/PSY.0b013e31822232bb
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Scharff, A, Ortiz, SN, Forrest, LN, and Smith, AR. Comparing the clinical presentation of eating disorder patients with and without trauma history and/or comorbid PTSD. Eat Disord. (2019) 29:88–102. doi: 10.1080/10640266.2019.1642035
CrossRef Full Text | Google Scholar
7. Dansky, BS, Brewerton, TD, Kilpatrick, DG, and O’Neil, PM. The National Women’s study: relationship of victimization and posttraumatic stress disorder to bulimia nervosa. Int J Eat Disord. (1997) 21:213–28. doi: 10.1002/(SICI)1098-108X(199704)21:33.0.CO;2-N
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Ferrell, EL, Russin, SE, and Flint, DD. Prevalence estimates of comorbid eating disorders and posttraumatic stress disorder: a quantitative synthesis. J Aggress Maltreat Trauma. (2020) 31:264–82. doi: 10.1080/10926771.2020.1832168
CrossRef Full Text | Google Scholar
9. Hudson, JI, Hiripi, E, Pope, HG Jr, and Kessler, RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. (2007) 61:348–58. doi: 10.1016/j.biopsych.2006.03.040
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Brewerton, TD, Gavidia, I, Suro, G, Perlman, MM, Genet, J, and Bunnell, DW. Provisional posttraumatic stress disorder is associated with greater severity of eating disorder and comorbid symptoms in adolescents treated in residential care. Eur Eat Disord Rev. (2021) 29:910–23. doi: 10.1002/erv.2864
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Scharff, A, Ortiz, SN, Forrest, LN, Smith, AR, and Boswell, JF. Post-traumatic stress disorder as a moderator of transdiagnostic, residential eating disorder treatment outcome trajectory. J Clin Psychol. (2021) 77:986–1003. doi: 10.1002/jclp.23106
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Brewerton, TD, Wang, JB, Lafrance, A, Pamplin, C, Mithoefer, M, Yazar-Klosinki, B, et al. MDMA-assisted therapy significantly reduces eating disorder symptoms in a randomized placebo-controlled trial of adults with severe PTSD. J Psychiatr Res. (2022) 149:128–35. doi: 10.1016/j.jpsychires.2022.03.008
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Brewerton, TD, Perlman, MM, Gavidia, I, Suro, G, and Jahraus, J. Headache, eating disorders, PTSD, and comorbidity: implications for assessment and treatment. Eat Weight Disord. (2022) 27:2693–700. doi: 10.1007/s40519-022-01414-6
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Afifi, TO, Sareen, J, Fortier, J, Taillieu, T, Turner, S, Cheung, K, et al. Child maltreatment and eating disorders among men and women in adulthood: results from a nationally representative United States sample. Int J Eat Disord. (2017) 50:1281–96. doi: 10.1002/eat.22783
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Brewerton, T. D., and Dennis, A. B. (2015). Perpetuating factors in severe and enduring anorexia nervosa. In S. Touyz, P. Hay, D. GrangeLe, and J. H. Lacey (Eds.), Managing severe and enduring anorexia nervosa: a clinician’s handbook. New York, NY: Routledge.
Google Scholar
16. Molendijk, ML, Hoek, HW, Brewerton, TD, and Elzinga, BM. Childhood maltreatment and eating disorder pathology: a systematic review and dose-response meta-analysis. Psychol Med. (2017) 47:1402–16. doi: 10.1017/S0033291716003561
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Anderson, KP, LaPorte, DJ, Brandt, H, and Crawford, S. Sexual abuse and bulimia: response to inpatient treatment and preliminary outcome. J Psychiatr Res. (1997) 31:621–33. doi: 10.1016/S0022-3956(97)00026-5
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Castellini, G, Lelli, L, Cassioli, E, Ciampi, E, Zamponi, F, Campone, B, et al. Different outcomes, psychopathological features, and comorbidities in patients with eating disorders reporting childhood abuse: a 3-year follow-up study. Eur Eat Disord Rev. (2018) 26:217–29. doi: 10.1002/erv.2586
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Convertino, A. D., and Mendoza, R. R. (2023). Posttraumatic stress disorder, traumatic events, and longitudinal eating disorder treatment outcomes: a systematic review. Int J Eat Disord doi:doi: 10.1002/eat.23933 [Epub ahead of print]
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Fallon, BA, Sadik, C, Saoud, JB, and Garfinkel, RS. Childhood abuse, family environment, and outcome in bulimia nervosa. J Clin Psychiatry. (1994) 55:424–8.
PubMed Abstract | Google Scholar
21. Hazzard, VM, Crosby, RD, Crow, SJ, Engel, SG, Schaefer, LM, Brewerton, TD, et al. Treatment outcomes of psychotherapy for binge-eating disorder in a randomized controlled trial: examining the roles of childhood abuse and post-traumatic stress disorder. Eur Eat Disord Rev. (2021) 29:611–21. doi: 10.1002/erv.2823
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Mahon, J, Bradley, SN, Harvey, PK, Winston, AP, and Palmer, RL. Childhood trauma has dose effect relationship with dropping out from psychotherapeutic treatment for bulimia nervosa: a replication. Int J Eat Disord. (2001) 30:138–48. doi: 10.1002/eat.1066
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Rodriguez, M, Perez, V, and Garcia, Y. Impact of traumatic experiences and violent acts upon response to treatment of a sample of Colombian women with eating disorders. Int J Eat Disord. (2005) 37:299–306. doi: 10.1002/eat.20091
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Serra, R, Kiekens, G, Tarsitani, L, Vrieze, E, Bruffaerts, R, Loriedo, C, et al. The effect of trauma and dissociation on the outcome of cognitive behavioural therapy for binge eating disorder: a 6-month prospective study. Eur Eat Disord Rev. (2020) 28:309–17. doi: 10.1002/erv.2722
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Trottier, K. Posttraumatic stress disorder predicts non-completion of day hospital treatment for bulimia nervosa and other specified feeding/eating disorder. Eur Eat Disord Rev. (2020) 28:343–50. doi: 10.1002/erv.2723
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Vrabel, KR, Hoffart, A, Ro, O, Martinsen, EW, and Rosenvinge, JH. Co-occurrence of avoidant personality disorder and child sexual abuse predicts poor outcome in long-standing eating disorder. J Abnorm Psychol. (2010) 119:623–9. doi: 10.1037/a0019857
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Brewerton, TD, Cotton, BD, and Kilpatrick, DG. Sensation seeking, binge-type eating disorders, victimization, and PTSD in the National Women’s study. Eat Behav. (2018) 30:120–4. doi: 10.1016/j.eatbeh.2018.07.001
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Mitchell, JM, Bogenschutz, M, Lilienstein, A, Harrison, C, Kleiman, S, Parker-Guilbert, K, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. (2021) 27:1025–33. doi: 10.1038/s41591-021-01336-3
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Mitchell, KS, Wells, SY, Mendes, A, and Resick, PA. Treatment improves symptoms shared by PTSD and disordered eating. J Trauma Stress. (2012) 25:535–42. doi: 10.1002/jts.21737
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Vierling, V, Etori, S, Valenti, L, Lesage, M, Pigeyre, M, Dodin, V, et al. Prevalence and impact of post-traumatic stress disorder in a disordered eating population sample. Presse Med. (2015) 44:e341–52. doi: 10.1016/j.lpm.2015.04.039
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Kessler, RC, Aguilar-Gaxiola, S, Alonso, J, Benjet, C, Bromet, EJ, Cardoso, G, et al. Trauma and PTSD in the WHO world mental health surveys. Eur J Psychotraumatol. (2017) 8:1353383. doi: 10.1080/20008198.2017.1353383
CrossRef Full Text | Google Scholar
32. Kessler, RC, Sonnega, A, Bromet, E, Hughes, M, and Nelson, CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. (1995) 52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Koenen, KC, Ratanatharathorn, A, Ng, L, McLaughlin, KA, Bromet, EJ, Stein, DJ, et al. Posttraumatic stress disorder in the world mental health surveys. Psychol Med. (2017) 47:2260–74. doi: 10.1017/S0033291717000708
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Hilbert, A, Hoek, HW, and Schmidt, R. Evidence-based clinical guidelines for eating disorders: international comparison. Curr Opin Psychiatry. (2017) 30:423–37. doi: 10.1097/YCO.0000000000000360
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Hay, P, Chinn, D, Forbes, D, Madden, S, Newton, R, Sugenor, L, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of eating disorders. Aust N Z J Psychiatry. (2014) 48:977–1008. doi: 10.1177/0004867414555814
PubMed Abstract | CrossRef Full Text | Google Scholar
37. American Psychiatric Association (2023). The American Psychiatric Association practice guideline for the treatment of patients with eating disorders. Washington, DC: American Psychiatric Association Publishing.
Google Scholar
38. Crone, C, Fochtmann, LJ, Attia, E, Boland, R, Escobar, J, Fornari, V, et al. The American Psychiatric Association practice guideline for the treatment of patients with eating disorders. Am J Psychiatry. (2023) 180:167–71. doi: 10.1176/appi.ajp.23180001
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Ralph, AF, Brennan, L, Byrne, S, Caldwell, B, Farmer, J, Hart, LM, et al. Management of eating disorders for people with higher weight: clinical practice guideline. J Eat Disord. (2022) 10:121. doi: 10.1186/s40337-022-00622-w
CrossRef Full Text | Google Scholar
40. Longo, P, Marzola, E, De Bacco, C, Demarchi, M, and Abbate-Daga, G. Young patients with anorexia nervosa: the contribution of post-traumatic stress disorder and traumatic events. Medicina. (2020) 34:665–74. doi: 10.3390/medicina57010002
CrossRef Full Text | Google Scholar
41. Couturier, J, Isserlin, L, Norris, M, Spettigue, W, Brouwers, M, Kimber, M, et al. Canadian practice guidelines for the treatment of children and adolescents with eating disorders. J Eat Disord. (2020) 8:4. doi: 10.1186/s40337-020-0277-8
CrossRef Full Text | Google Scholar
42. Ebeling, H, Tapanainen, P, Joutsenoja, A, Koskinen, M, Morin-Papunen, L, Jarvi, L, et al. A practice guideline for treatment of eating disorders in children and adolescents. Ann Med. (2003) 35:488–501. doi: 10.1080/07853890310000727
CrossRef Full Text | Google Scholar
43. Lock, J, and La Via, MC, American Academy of, C., & Adolescent Psychiatry Committee on Quality, I. Practice parameter for the assessment and treatment of children and adolescents with eating disorders. J Am Acad Child Adolesc Psychiatry. (2015) 54:412–25. doi: 10.1016/j.jaac.2015.01.018
CrossRef Full Text | Google Scholar
44. Berliner, L., Bisson, J., Cloitre, M., Forbes, D., Goldbeck, L., Jensen, T. K., et al. (2017). ISTSS guidelines position paper on complex PTSD in children and adolescents, Oakbrook Terrace, IL: International Society for Traumatic Stress Studies.
Google Scholar
45. Bisson, JI, Berliner, L, Cloitre, M, Forbes, D, Jensen, TK, Lewis, C, et al. The International Society for Traumatic Stress Studies new Guidelines for the prevention and treatment of posttraumatic stress disorder: methodology and development process. J Trauma Stress. (2019) 32:475–83. doi: 10.1002/jts.22421
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Cloitre, M., Courtois, C. A., Ford, J. D., Green, B. L., Alexander, P., Briere, J., et al. (2012). ISTSS expert consensus guidelines for complex PTSD, Oakbrook Terrace, IL: International Society for Traumatic Stress Studies.
Google Scholar
47. Foa, EB, Keane, TM, Friedman, MJ, and Cohen, JA. Effective treatments for PTSD: Practice guidelines from the International Society for Traumatic Stress Studies. Second ed New York, NY: The Guilford Press (2009).
Google Scholar
48. Forbes, D, Creamer, M, Bisson, JI, Cohen, JA, Crow, BE, Foa, EB, et al. A guide to guidelines for the treatment of PTSD and related conditions. J Trauma Stress. (2010) 23:537–52. doi: 10.1002/jts.20565
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Guideline Development Panel for the Treatment of PTSD in Adults, A. P. A. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. (2019) 74:596–607. doi: 10.1037/amp0000473
PubMed Abstract | CrossRef Full Text | Google Scholar
50. Lewis, C, Roberts, NP, Andrew, M, Starling, E, and Bisson, JI. Psychological therapies for post-traumatic stress disorder in adults: systematic review and meta-analysis. Eur J Psychotraumatol. (2020) 11:1729633. doi: 10.1080/20008198.2020.1729633
CrossRef Full Text | Google Scholar
51. National Institute for Health and Care Excellence (NICE). (2018). Guideline for posttraumatic stress disorder. National Institute for Health and Clinical Practice.
Google Scholar
52. Phelps, AJ, Lethbridge, R, Brennan, S, Bryant, RA, Burns, P, Cooper, JA, et al. Australian guidelines for the prevention and treatment of posttraumatic stress disorder: updates in the third edition. Aust N Z J Psychiatry. (2022) 56:230–47. doi: 10.1177/00048674211041917
PubMed Abstract | CrossRef Full Text | Google Scholar
53. The Expert Concensus Panels for PTSD. The expert consensus guideline series. Treatment of posttraumatic stress disorder PTSD. J Clin Psychiatry. (1999) 60:3–76.
Google Scholar
55. Ursano, RJ, Bell, C, Eth, S, Friedman, M, Norwood, A, Pfefferbaum, B, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry. (2004) 161:3–31.
PubMed Abstract | Google Scholar
56. Hamblen, JL, Norman, SB, Sonis, JH, Phelps, AJ, Bisson, JI, Nunes, VD, et al. A guide to guidelines for the treatment of posttraumatic stress disorder in adults: An update. Psychotherapy. (2019) 56:359–73. doi: 10.1037/pst0000231
PubMed Abstract | CrossRef Full Text | Google Scholar
57. Brewerton, TD. Eating disorders, victimization, and comorbidity: principles of treatment In: TD Brewerton, editor. Clinical handbook of eating disorders: an integrated approach : New York, NY: Marcel Decker (2004). 509–45.
Google Scholar
58. Trim, JG, Galovski, T, Wagner, A, and Brewerton, TD. Treating eating disorder-PTSD patients: a synthesis of the literature and new treatment directions In: LK Anderson, SB Murray, and WH Kaye, editors. Clinical handbook of complex and atypical eating disorders : New York, NY: Oxford University Press (2017). 40–59.
Google Scholar
59. Ben-Shlomo, Y, and Kuh, D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. (2002) 31:285–93. doi: 10.1093/ije/31.2.285
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Fried, EI, and Cramer, AOJ. Moving forward: challenges and directions for psychopathological network theory and methodology. Perspect Psychol Sci. (2017) 12:999–1020. doi: 10.1177/1745691617705892
PubMed Abstract | CrossRef Full Text | Google Scholar
61. Fried, EI, van Borkulo, CD, Cramer, AO, Boschloo, L, Schoevers, RA, and Borsboom, D. Mental disorders as networks of problems: a review of recent insights. Soc Psychiatry Psychiatr Epidemiol. (2017) 52:1–10. doi: 10.1007/s00127-016-1319-z
PubMed Abstract | CrossRef Full Text | Google Scholar
64. Olofsson, ME, Oddli, HW, Hoffart, A, Eielsen, HP, and Vrabel, KR. Change processes related to long-term outcomes in eating disorders with childhood trauma: An explorative qualitative study. J Couns Psychol. (2020) 67:51–65. doi: 10.1037/cou0000375
PubMed Abstract | CrossRef Full Text | Google Scholar
65. Olofsson, ME, Vrabel, KR, Hoffart, A, and Oddli, HW. Covert therapeutic micro-processes in non-recovered eating disorders with childhood trauma: an interpersonal process recall study. J Eat Disord. (2022) 10:42. doi: 10.1186/s40337-022-00566-1
CrossRef Full Text | Google Scholar
66. Cramer, MA. Under the influence of unconscious process: countertransference in the treatment of PTSD and substance abuse in women. Am J Psychother. (2002) 56:194–210. doi: 10.1176/appi.psychotherapy.2002.56.2.194
PubMed Abstract | CrossRef Full Text | Google Scholar
67. Satir, DA, Thompson-Brenner, H, Boisseau, CL, and Crisafulli, MA. Countertransference reactions to adolescents with eating disorders: relationships to clinician and patient factors. Int J Eat Disord. (2009) 42:511–21. doi: 10.1002/eat.20650
PubMed Abstract | CrossRef Full Text | Google Scholar
68. Thompson-Brenner, H, Satir, DA, Franko, DL, and Herzog, DB. Clinician reactions to patients with eating disorders: a review of the literature. Psychiatr Serv. (2012) 63:73–8. doi: 10.1176/appi.ps.201100050
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Resick, PA, Monson, CM, and Chard, KM. Cognitive processing therapy for PTSD: A comprehensive manual, New York, NY: The Guilford Press (2017).
Google Scholar
70. Cavicchioli, M, Scalabrini, A, Northoff, G, Mucci, C, Ogliari, A, and Maffei, C. Dissociation and emotion regulation strategies: a meta-analytic review. J Psychiatr Res. (2021) 143:370–87. doi: 10.1016/j.jpsychires.2021.09.011
PubMed Abstract | CrossRef Full Text | Google Scholar
71. Daneshvar, S, Shafiei, M, and Basharpoor, S. Group-based compassion-focused therapy on experiential avoidance, meaning-in-life, and sense of coherence in female survivors of intimate partner violence with PTSD: a randomized controlled trial. J Interpers Viol. (2022) 37:NP4187–211. doi: 10.1177/0886260520958660
CrossRef Full Text | Google Scholar
72. Della Longa, NM, and De Young, KP. Experiential avoidance, eating expectancies, and binge eating: a preliminary test of an adaption of the acquired preparedness model of eating disorder risk. Appetite. (2018) 120:423–30. doi: 10.1016/j.appet.2017.09.022
PubMed Abstract | CrossRef Full Text | Google Scholar
73. Fulton, JJ, Lavender, JM, Tull, MT, Klein, AS, Muehlenkamp, JJ, and Gratz, KL. The relationship between anxiety sensitivity and disordered eating: the mediating role of experiential avoidance. Eat Behav. (2012) 13:166–9. doi: 10.1016/j.eatbeh.2011.12.003
PubMed Abstract | CrossRef Full Text | Google Scholar
74. Oldershaw, A, Lavender, T, Sallis, H, Stahl, D, and Schmidt, U. Emotion generation and regulation in anorexia nervosa: a systematic review and meta-analysis of self-report data. Clin Psychol Rev. (2015) 39:83–95. doi: 10.1016/j.cpr.2015.04.005
PubMed Abstract | CrossRef Full Text | Google Scholar
75. Wooldridge, JS, Herbert, MS, Dochat, C, and Afari, N. Understanding relationships between posttraumatic stress disorder symptoms, binge-eating symptoms, and obesity-related quality of life: the role of experiential avoidance. Eat Disord. (2021) 29:260–75. doi: 10.1080/10640266.2020.1868062
PubMed Abstract | CrossRef Full Text | Google Scholar
76. Brady, KT, Killeen, TK, Brewerton, T, and Lucerini, S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. (2000) 61:22–32.
Google Scholar
78. Hambleton, A, Pepin, G, Le, A, Maloney, D, National Eating Disorder Research, C, Touyz, S, et al. Psychiatric and medical comorbidities of eating disorders: findings from a rapid review of the literature. J Eat Disord. (2022) 10:132. doi: 10.1186/s40337-022-00654-2
CrossRef Full Text | Google Scholar
79. Goodnight, JRM, Ragsdale, KA, Rauch, SAM, and Rothbaum, BO. Psychotherapy for PTSD: An evidence-based guide to a theranostic approach to treatment. Prog Neuro-Psychopharmacol Biol Psychiatry. (2019) 88:418–26. doi: 10.1016/j.pnpbp.2018.05.006
PubMed Abstract | CrossRef Full Text | Google Scholar
80. van Minnen, A, Harned, MS, Zoellner, L, and Mills, K. Examining potential contraindications for prolonged exposure therapy for PTSD. Eur J Psychotraumatol. (2012) 2012:3. doi: 10.3402/ejpt.v3i0.18805
CrossRef Full Text | Google Scholar
81. Mills, KL, Teesson, M, Back, SE, Brady, KT, Baker, AL, Hopwood, S, et al. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: a randomized controlled trial. JAMA. (2012) 308:690–9. doi: 10.1001/jama.2012.9071
PubMed Abstract | CrossRef Full Text | Google Scholar
82. Ronconi, JM, Shiner, B, and Watts, BV. Inclusion and exclusion criteria in randomized controlled trials of psychotherapy for PTSD. J Psychiatr Pract. (2014) 20:25–37. doi: 10.1097/01.pra.0000442936.23457.5b
PubMed Abstract | CrossRef Full Text | Google Scholar
83. Ruglass, LM, Lopez-Castro, T, Papini, S, Killeen, T, Back, SE, and Hien, DA. Concurrent treatment with prolonged exposure for co-occurring full or subthreshold posttraumatic stress disorder and substance use disorders: a randomized clinical trial. Psychother Psychosom. (2017) 86:150–61. doi: 10.1159/000462977
PubMed Abstract | CrossRef Full Text | Google Scholar
84. Cloitre, M, Courtois, CA, Charuvastra, A, Carapezza, R, Stolbach, BC, and Green, BL. Treatment of complex PTSD: results of the ISTSS expert clinician survey on best practices. J Trauma Stress. (2011) 24:615–27. doi: 10.1002/jts.20697
PubMed Abstract | CrossRef Full Text | Google Scholar
85. Cloitre, M, Petkova, E, Wang, J, and Lu Lassell, F. An examination of the influence of a sequential treatment on the course and impact of dissociation among women with PTSD related to childhood abuse. Depress Anxiety. (2012) 29:709–17. doi: 10.1002/da.21920
PubMed Abstract | CrossRef Full Text | Google Scholar
86. Cloitre, M, Stovall-McClough, KC, Nooner, K, Zorbas, P, Cherry, S, Jackson, CL, et al. Treatment for PTSD related to childhood abuse: a randomized controlled trial. Am J Psychiatry. (2010) 167:915–24. doi: 10.1176/appi.ajp.2010.09081247
PubMed Abstract | CrossRef Full Text | Google Scholar
89. Rorty, M, and Yager, J. Histories of childhood trauma and complex post-traumatic sequelae in women with eating disorders. Psychiatr Clin North Am. (1996) 19:773–91. doi: 10.1016/S0193-953X(05)70381-6
PubMed Abstract | CrossRef Full Text | Google Scholar
90. Van der Kolk, BA. Assessment and treatment of complex PTSD In: R Yehuda, editor. Treating trauma survivors with PTSD (pp. 127–156) : Washington, DC: American Psychiatric Association Publishing (2002)
Google Scholar
91. Davis, ML, Fletcher, T, McIngvale, E, Cepeda, SL, Schneider, SC, La Buissonniere Ariza, V, et al. Clinicians’ perspectives of interfering behaviors in the treatment of anxiety and obsessive-compulsive disorders in adults and children. Cogn Behav Ther. (2020) 49:81–96. doi: 10.1080/16506073.2019.1579857
PubMed Abstract | CrossRef Full Text | Google Scholar
92. Federici, A, and Wisniewski, L. An intensive DBT program for patients with multidiagnostic eating disorder presentations: a case series analysis. Int J Eat Disord. (2013) 46:322–31. doi: 10.1002/eat.22112
PubMed Abstract | CrossRef Full Text | Google Scholar
93. Federici, A, Wisniewski, L, and Ben-Porath, D. Description of an intensive dialectical behavior therapy program for multidiagnostic clients with eating disorders. J Couns Dev. (2012) 90:330–8. doi: 10.1002/j.1556-6676.2012.00041.x
CrossRef Full Text | Google Scholar
94. De Jongh, A, Resick, PA, Zoellner, LA, van Minnen, A, Lee, CW, Monson, CM, et al. Critical analysis of the current treatment guidelines for complex PTSD in adults. Depress Anxiety. (2016) 33:359–69. doi: 10.1002/da.22469
PubMed Abstract | CrossRef Full Text | Google Scholar
96. Brewerton, TD. Mechanisms by which adverse childhood experiences, other traumas and PTSD influence the health and well-being of individuals with eating disorders throughout the life span. J Eat Disord. (2022) 10:162. doi: 10.1186/s40337-022-00696-6
CrossRef Full Text | Google Scholar
97. Liebman, RE, Becker, KR, Smith, KE, Cao, L, Keshishian, AC, Crosby, RD, et al. Network analysis of posttraumatic stress and eating disorder symptoms in a community sample of adults exposed to childhood abuse. J Trauma Stress. (2020) 34:665–74. doi: 10.1002/jts.22644
CrossRef Full Text | Google Scholar
98. Monteleone, AM, Tzischinsky, O, Cascino, G, Alon, S, Pellegrino, F, Ruzzi, V, et al. The connection between childhood maltreatment and eating disorder psychopathology: a network analysis study in people with bulimia nervosa and with binge eating disorder. Eat Weight Disord. (2022) 27:253–61. doi: 10.1007/s40519-021-01169-6
PubMed Abstract | CrossRef Full Text | Google Scholar
99. Nelson, JD, Cuellar, AE, Cheskin, LJ, and Fischer, S. Eating disorders and post-traumatic stress disorder: a network analysis of the comorbidity. Behav Ther. (2021) 53:310–22. doi: 10.1016/j.beth.2021.09.006
CrossRef Full Text | Google Scholar
100. Vanzhula, IA, Calebs, B, Fewell, L, and Levinson, CA. Illness pathways between eating disorder and post-traumatic stress disorder symptoms: understanding comorbidity with network analysis. Eur Eat Disord Rev. (2019) 27:147–60. doi: 10.1002/erv.2634
PubMed Abstract | CrossRef Full Text | Google Scholar
101. Cook, JM, Simiola, V, Hamblen, JL, Bernardy, N, and Schnurr, PP. The influence of patient readiness on implementation of evidence-based PTSD treatments in veterans affairs residential programs. Psychol Trauma. (2017) 9:51–8. doi: 10.1037/tra0000162
CrossRef Full Text | Google Scholar
102. Fetahi, E, Sogaard, AS, and Sjogren, M. Estimating the effect of motivational interventions in patients with eating disorders: a systematic review and Meta-analysis. J Pers Med. (2022) 12:577. doi: 10.3390/jpm12040577
PubMed Abstract | CrossRef Full Text | Google Scholar
103. Geller, J, Brown, KE, and Srikameswaran, S. The efficacy of a brief motivational intervention for individuals with eating disorders: a randomized control trial. Int J Eat Disord. (2011) 44:497–505. doi: 10.1002/eat.20847
PubMed Abstract | CrossRef Full Text | Google Scholar
104. Jakupcak, M, Hoerster, KD, Blais, RK, Malte, CA, Hunt, S, and Seal, K. Readiness for change predicts VA mental healthcare utilization among Iraq and Afghanistan war veterans. J Trauma Stress. (2013) 26:165–8. doi: 10.1002/jts.21768
PubMed Abstract | CrossRef Full Text | Google Scholar
105. Kaysen, D, Jaffe, AE, Shoenberger, B, Walton, TO, Pierce, AR, and Walker, DD. Does effectiveness of a brief substance use treatment depend on PTSD? An evaluation of motivational enhancement therapy for active-duty Army personnel. J Stud Alcohol Drugs. (2022) 83:924–33. doi: 10.15288/jsad.22-00011
PubMed Abstract | CrossRef Full Text | Google Scholar
106. Killeen, TK, Cassin, SE, and Geller, J. Motivational interviewing in the treatment of substance use disorders, addictions, and eating disorders In: TD Brewerton and AB Dennis, editors. Eating Disorders, addictions and substance use Disorders: Research, Clinical and Treratment Perspectives : Berlin, Germany: Springer (2014)
Google Scholar
107. Vitousek, K, Watson, S, and Wilson, GT. Enhancing motivation for change in treatment-resistant eating disorders. Clin Psychol Rev. (1998) 18:391–420. doi: 10.1016/S0272-7358(98)00012-9
PubMed Abstract | CrossRef Full Text | Google Scholar
108. Weiss, CV, Mills, JS, Westra, HA, and Carter, JC. A preliminary study of motivational interviewing as a prelude to intensive treatment for an eating disorder. J Eat Disord. (2013) 1:34. doi: 10.1186/2050-2974-1-34
CrossRef Full Text | Google Scholar
109. Chuemere, AN, Oluwatayo, BO, Kolawole, TA, and Ilochi, ON. Starvation-induced changes in memory sensitization, habituation and psychosomatic responses. Int J Trop Dis Health. (2019) 35:1–7. doi: 10.9734/ijtdh/2019/v35i330126
CrossRef Full Text | Google Scholar
110. Park, SB, Coull, JT, McShane, RH, Young, AH, Sahakian, BJ, Robbins, TW, et al. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. (1994) 33:575–88. doi: 10.1016/0028-3908(94)90089-2
CrossRef Full Text | Google Scholar
111. Riedel, WJ, Klaassen, T, Deutz, NE, van Someren, A, and van Praag, HM. Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology. (1999) 141:362–9. doi: 10.1007/s002130050845
PubMed Abstract | CrossRef Full Text | Google Scholar
112. Brewerton, TD, Gavidia, I, Suro, G, and Perlman, MM. Eating disorder patients with and without PTSD treated in residential care: discharge and 6-month follow-up results. J Eat Disord. (2023) 11:48. doi: 10.1186/s40337-023-00773-4
CrossRef Full Text | Google Scholar
113. Trottier, K, and Monson, CM. Integrating cognitive processing therapy for posttraumatic stress disorder with cognitive behavioral therapy for eating disorders in PROJECT RECOVER. Eat Disord. (2021) 29:307–25. doi: 10.1080/10640266.2021.1891372
PubMed Abstract | CrossRef Full Text | Google Scholar
114. Brewin, N, Baggott, J, Dugard, P, and Arcelus, J. Clinical normative data for eating disorder examination questionnaire and eating disorder inventory for DSM-5 feeding and eating disorder classifications: a retrospective study of patients formerly diagnosed via DSM-IV. Eur Eat Disord Rev. (2014) 22:299–305. doi: 10.1002/erv.2301
PubMed Abstract | CrossRef Full Text | Google Scholar
115. Blevins, CA, Weathers, FW, Davis, MT, Witte, TK, and Domino, JL. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. (2015) 28:489–98. doi: 10.1002/jts.22059
PubMed Abstract | CrossRef Full Text | Google Scholar
116. Des Jarlais, DC. Harm reduction in the USA: the research perspective and an archive to David purchase. Harm Reduct J. (2017) 14:51. doi: 10.1186/s12954-017-0178-6
CrossRef Full Text | Google Scholar
118. Yager, J. Why defend harm reduction for severe and enduring eating Disorders? Who Wouldn’t want to reduce harms? Am J Bioeth. (2021) 21:57–9. doi: 10.1080/15265161.2021.1926160
PubMed Abstract | CrossRef Full Text | Google Scholar
119. Brewerton, TD. Posttraumatic stress disorder and disordered eating: food addiction as self-medication. J Women’s Health (Larchmt). (2011) 20:1133–4. doi: 10.1089/jwh.2011.3050
CrossRef Full Text | Google Scholar
120. Kearney-Cooke, A, and Striegel-Moore, RH. Treatment of childhood sexual abuse in anorexia nervosa and bulimia nervosa: a feminist psychodynamic approach. Int J Eat Disord. (1994) 15:305–19. doi: 10.1002/eat.2260150402
PubMed Abstract | CrossRef Full Text | Google Scholar
121. Brewerton, T. D., Trottier, K., Trim, J. G., Myers, T., and Wonderlich, S. (2020). Integrating evidence-based treatments for eating disorder patients with comorbid PTSD and trauma-related Disorders. In C. C. Tortolani, A. B. Goldschmidt, and D. GrangeLe (Eds.), Adapting evidence-based treatments for eating Disorders for novel populations and settings: A practical guide (pp. 216–237). New York, NY: Routledge.
Google Scholar
122. Trottier, K, Monson, CM, Wonderlich, SA, and Crosby, RD. Results of the first randomized controlled trial of integrated cognitive-behavioral therapy for eating disorders and posttraumatic stress disorder. Psychol Med. (2022) 52:587–96. doi: 10.1017/S0033291721004967
PubMed Abstract | CrossRef Full Text | Google Scholar
123. Trottier, K, Monson, CM, Wonderlich, SA, and Olmsted, MP. Initial findings from project recover: overcoming co-occurring eating disorders and posttraumatic stress disorder through integrated treatment. J Trauma Stress. (2017) 30:173–7. doi: 10.1002/jts.22176
PubMed Abstract | CrossRef Full Text | Google Scholar
124. Bloomgarden, A, and Calogero, RM. A randomized experimental test of the efficacy of EMDR treatment on negative body image in eating disorder inpatients. Eat Disord. (2008) 16:418–27. doi: 10.1080/10640260802370598
PubMed Abstract | CrossRef Full Text | Google Scholar
125. Bongaerts, H, Van Minnen, A, and de Jongh, A. Intensive EMDR to treat patients with complex posttraumatic stress disorder: a case series. J EMDR Pract Res. (2017) 11:84–95. doi: 10.1891/1933-3196.11.2.84
CrossRef Full Text | Google Scholar
126. Boterhoven de Haan, KL, Lee, CW, Fassbinder, E, van Es, SM, Menninga, S, Meewisse, ML, et al. Imagery rescripting and eye movement desensitisation and reprocessing as treatment for adults with post-traumatic stress disorder from childhood trauma: randomised clinical trial. Br J Psychiatry. (2020) 217:609–15. doi: 10.1192/bjp.2020.158
PubMed Abstract | CrossRef Full Text | Google Scholar
127. Claudat, K, Reilly, EE, Convertino, AD, Trim, J, Cusack, A, and Kaye, WH. Integrating evidence-based PTSD treatment into intensive eating disorders treatment: a preliminary investigation. Eat Weight Disord. (2022) 27:3599–607. doi: 10.1007/s40519-022-01500-9
PubMed Abstract | CrossRef Full Text | Google Scholar
128. Cuijpers, P, Veen, SCV, Sijbrandij, M, Yoder, W, and Cristea, IA. Eye movement desensitization and reprocessing for mental health problems: a systematic review and meta-analysis. Cogn Behav Ther. (2020) 49:165–80. doi: 10.1080/16506073.2019.1703801
PubMed Abstract | CrossRef Full Text | Google Scholar
129. Daneshvar, S, Shafiei, M, and Basharpoor, S. Compassion-focused therapy: proof of concept trial on suicidal ideation and cognitive distortions in female survivors of intimate partner violence with PTSD. J Interpers Viol. (2022) 37:NP9613–34. doi: 10.1177/0886260520984265
CrossRef Full Text | Google Scholar
131. Irons, C, and Lad, S. Using compassion focused therapy to work with shame and self-criticism in complex PTSD. Aust Clin Psychol. (2017) 3:47–54.
Google Scholar
132. Kelly, AC, Wisniewski, L, Martin-Wagar, C, and Hoffman, E. Group-based compassion-focused therapy as an adjunct to outpatient treatment for eating disorders: a pilot randomized controlled trial. Clin Psychol Psychother. (2017) 24:475–87. doi: 10.1002/cpp.2018
PubMed Abstract | CrossRef Full Text | Google Scholar
133. Lawrence, VA, and Lee, D. An exploration of people’s experiences of compassion-focused therapy for trauma, using interpretative phenomenological analysis. Clin Psychol Psychother. (2014) 21:495–507. doi: 10.1002/cpp.1854
PubMed Abstract | CrossRef Full Text | Google Scholar
134. Lely, JCG, Smid, GE, Jongedijk, RA, Knipscheer, JW, and Kleber, RJ. The effectiveness of narrative exposure therapy: a review, meta-analysis and meta-regression analysis. Eur J Psychotraumatol. (2019) 10:1550344. doi: 10.1080/20008198.2018.1550344
CrossRef Full Text | Google Scholar
135. Luo, X, Che, X, Lei, Y, and Li, H. Investigating the influence of self-compassion-focused interventions on posttraumatic stress: a systematic review and Meta-analysis. Mindfulness. (2021) 12:2865–76. doi: 10.1007/s12671-021-01732-3
PubMed Abstract | CrossRef Full Text | Google Scholar
137. Moreno-Alcazar, A, Treen, D, Valiente-Gomez, A, Sio-Eroles, A, Perez, V, Amann, BL, et al. Efficacy of eye movement desensitization and reprocessing in children and adolescent with post-traumatic stress disorder: a meta-analysis of randomized controlled trials. Front Psychol. (2017) 8:1750. doi: 10.3389/fpsyg.2017.01750
PubMed Abstract | CrossRef Full Text | Google Scholar
138. Morkved, N, Hartmann, K, Aarsheim, LM, Holen, D, Milde, AM, Bomyea, J, et al. A comparison of narrative exposure therapy and prolonged exposure therapy for PTSD. Clin Psychol Rev. (2014) 34:453–67. doi: 10.1016/j.cpr.2014.06.005
PubMed Abstract | CrossRef Full Text | Google Scholar
139. Rothbaum, BO, Astin, MC, and Marsteller, F. Prolonged exposure versus eye movement desensitization and reprocessing (EMDR) for PTSD rape victims. J Trauma Stress. (2005) 18:607–16. doi: 10.1002/jts.20069
PubMed Abstract | CrossRef Full Text | Google Scholar
140. Sloan, DM, Marx, BP, Lee, DJ, and Resick, PA. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. (2018) 75:233–9. doi: 10.1001/jamapsychiatry.2017.4249
PubMed Abstract | CrossRef Full Text | Google Scholar
141. Ten Napel-Schutz, MC, Vroling, M, Mares, SHW, and Arntz, A. Treating PTSD with imagery rescripting in underweight eating disorder patients: a multiple baseline case series study. J Eat Disord. (2022) 10:35. doi: 10.1186/s40337-022-00558-1
CrossRef Full Text | Google Scholar
142. ten Napel-Schutz, MC, Karbouniaris, S, Mares, SHW, Arntz, A, and Abma, TA. Perspectives of underweight people with eating disorders on receiving imagery Rescripting trauma treatment: a qualitative study of their experiences. J Eat Disord. (2022) 10:188. doi: 10.1186/s40337-022-00712-9
CrossRef Full Text | Google Scholar
143. Fogelkvist, M, Gustafsson, SA, Kjellin, L, and Parling, T. Acceptance and commitment therapy to reduce eating disorder symptoms and body image problems in patients with residual eating disorder symptoms: a randomized controlled trial. Body Image. (2020) 32:155–66. doi: 10.1016/j.bodyim.2020.01.002
PubMed Abstract | CrossRef Full Text | Google Scholar
144. Juarascio, AS, Forman, EM, and Herbert, JD. Acceptance and commitment therapy versus cognitive therapy for the treatment of comorbid eating pathology. Behav Modif. (2010) 34:175–90. doi: 10.1177/0145445510363472
PubMed Abstract | CrossRef Full Text | Google Scholar
145. Linardon, J, Fairburn, CG, Fitzsimmons-Craft, EE, Wilfley, DE, and Brennan, L. The empirical status of the third-wave behaviour therapies for the treatment of eating disorders: a systematic review. Clin Psychol Rev. (2017) 58:125–40. doi: 10.1016/j.cpr.2017.10.005
PubMed Abstract | CrossRef Full Text | Google Scholar
146. Orsillo, SM, and Batten, SV. Acceptance and commitment therapy in the treatment of posttraumatic stress disorder. Behav Modif. (2005) 29:95–129. doi: 10.1177/0145445504270876
PubMed Abstract | CrossRef Full Text | Google Scholar
147. Back, M, Falkenstrom, F, Gustafsson, SA, Andersson, G, and Holmqvist, R. Reduction in depressive symptoms predicts improvement in eating disorder symptoms in interpersonal psychotherapy: results from a naturalistic study. J Eat Disord. (2020) 8:33. doi: 10.1186/s40337-020-00308-1
CrossRef Full Text | Google Scholar
148. Duberstein, PR, Ward, EA, Chaudron, LH, He, H, Toth, SL, Wang, W, et al. Effectiveness of interpersonal psychotherapy-trauma for depressed women with childhood abuse histories. J Consult Clin Psychol. (2018) 86:868–78. doi: 10.1037/ccp0000335
PubMed Abstract | CrossRef Full Text | Google Scholar
150. Markowitz, JC, Petkova, E, Neria, Y, Van Meter, PE, Zhao, Y, Hembree, E, et al. Is exposure necessary? A randomized clinical trial of interpersonal psychotherapy for PTSD. Am J Psychiatry. (2015) 172:430–40. doi: 10.1176/appi.ajp.2014.14070908
PubMed Abstract | CrossRef Full Text | Google Scholar
151. Schramm, E, Schneider, D, Zobel, I, van Calker, D, Dykierek, P, Kech, S, et al. Efficacy of interpersonal psychotherapy plus pharmacotherapy in chronically depressed inpatients. J Affect Disord. (2008) 109:65–73. doi: 10.1016/j.jad.2007.10.013
CrossRef Full Text | Google Scholar
152. Whiston, A, Bockting, CLH, and Semkovska, M. Towards personalising treatment: a systematic review and meta-analysis of face-to-face efficacy moderators of cognitive-behavioral therapy and interpersonal psychotherapy for major depressive disorder. Psychol Med. (2019) 49:2657–68. doi: 10.1017/S0033291719002812
PubMed Abstract | CrossRef Full Text | Google Scholar
153. Donovan, DM, Daley, DC, Brigham, GS, Hodgkins, CC, Perl, HI, Garrett, SB, et al. Stimulant abuser groups to engage in 12-step: a multisite trial in the National Institute on Drug Abuse clinical trials network. J Subst Abus Treat. (2013) 44:103–14. doi: 10.1016/j.jsat.2012.04.004
PubMed Abstract | CrossRef Full Text | Google Scholar
154. Kelly, JF, Abry, A, Ferri, M, and Humphreys, K. Alcoholics anonymous and 12-step facilitation treatments for alcohol use disorder: a distillation of a 2020 Cochrane review for clinicians and policy makers. Alcohol Alcohol. (2020) 55:641–51. doi: 10.1093/alcalc/agaa050
PubMed Abstract | CrossRef Full Text | Google Scholar
155. Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. (1997) 58:7–29. doi: 10.15288/jsa.1997.58.7
PubMed Abstract | CrossRef Full Text | Google Scholar
156. Mitchell, KS, Singh, S, Hardin, S, and Thompson-Brenner, H. The impact of comorbid posttraumatic stress disorder on eating disorder treatment outcomes: investigating the unified treatment model. Int J Eat Disord. (2021) 54:1260–9. doi: 10.1002/eat.23515
PubMed Abstract | CrossRef Full Text | Google Scholar
157. Church, D, Stapleton, P, Mollon, P, Feinstein, D, Boath, E, Mackay, D, et al. Guidelines for the treatment of PTSD using clinical EFT (emotional freedom techniques). Healthcare. (2018) 6:146. doi: 10.3390/healthcare6040146
PubMed Abstract | CrossRef Full Text | Google Scholar
158. Monteleone, AM, Pellegrino, F, Croatto, G, Carfagno, M, Hilbert, A, Treasure, J, et al. Treatment of eating disorders: a systematic meta-review of meta-analyses and network meta-analyses. Neurosci Biobehav Rev. (2022) 142:104857. doi: 10.1016/j.neubiorev.2022.104857
PubMed Abstract | CrossRef Full Text | Google Scholar
159. Sijercic, I, Liebman, RE, Ip, J, Whitfield, KM, Ennis, N, Sumantry, D, et al. A systematic review and meta-analysis of individual and couple therapies for posttraumatic stress disorder: clinical and intimate relationship outcomes. J Anxiety Disord. (2022) 91:102613. doi: 10.1016/j.janxdis.2022.102613
PubMed Abstract | CrossRef Full Text | Google Scholar
160. Bohus, M, Dyer, AS, Priebe, K, Kruger, A, Kleindienst, N, Schmahl, C, et al. Dialectical behaviour therapy for post-traumatic stress disorder after childhood sexual abuse in patients with and without borderline personality disorder: a randomised controlled trial. Psychother Psychosom. (2013) 82:221–33. doi: 10.1159/000348451
PubMed Abstract | CrossRef Full Text | Google Scholar
161. Harned, MS, Gallop, RJ, and Valenstein-Mah, HR. What changes when? The course of improvement during a stage-based treatment for suicidal and self-injuring women with borderline personality disorder and PTSD. Psychother Res. (2018) 28:761–75. doi: 10.1080/10503307.2016.1252865
CrossRef Full Text | Google Scholar
162. Bellehsen, M, Stoycheva, V, Cohen, BH, and Nidich, S. A pilot randomized controlled trial of transcendental meditation as treatment for posttraumatic stress disorder in veterans. J Trauma Stress. (2021) 35:22–31. doi: 10.1002/jts.22665
CrossRef Full Text | Google Scholar
163. Bormann, JE, Thorp, SR, Smith, E, Glickman, M, Beck, D, Plumb, D, et al. Individual treatment of posttraumatic stress disorder using Mantram repetition: a randomized clinical trial. Am J Psychiatry. (2018) 175:979–88. doi: 10.1176/appi.ajp.2018.17060611
PubMed Abstract | CrossRef Full Text | Google Scholar
164. Carei, TR, Fyfe-Johnson, AL, Breuner, CC, and Brown, MA. Randomized controlled clinical trial of yoga in the treatment of eating disorders. J Adolesc Health. (2010) 46:346–51. doi: 10.1016/j.jadohealth.2009.08.007
PubMed Abstract | CrossRef Full Text | Google Scholar
165. Foa, E, Hembree, E, and Rothbaum, BO. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences, Oxford, England: Oxford University Press (2007).
Google Scholar
166. Gallegos, AM, Crean, HF, Pigeon, WR, and Heffner, KL. Meditation and yoga for posttraumatic stress disorder: a meta-analytic review of randomized controlled trials. Clin Psychol Rev. (2017) 58:115–24. doi: 10.1016/j.cpr.2017.10.004
PubMed Abstract | CrossRef Full Text | Google Scholar
167. Haider, T, Chia-Liang, D, and Sharma, M. Efficacy of meditation-based interventions on posttraumatic stress disorder (PTSD) among veterans: a narrative review. Adv Mind Body Med. (2021) 35:16–24.
PubMed Abstract | Google Scholar
168. Hilton, L, Maher, AR, Colaiaco, B, Apaydin, E, Sorbero, ME, Booth, M, et al. Meditation for posttraumatic stress: systematic review and meta-analysis. Psychol Trauma. (2017) 9:453–60. doi: 10.1037/tra0000180
CrossRef Full Text | Google Scholar
169. Kelly, U, Haywood, T, Segell, E, and Higgins, M. Trauma-sensitive yoga for post-traumatic stress disorder in women veterans who experienced military sexual trauma: interim results from a randomized controlled trial. J Altern Complement Med. (2021) 27:S45–59. doi: 10.1089/acm.2020.0417
CrossRef Full Text | Google Scholar
170. Mavranezouli, I, Megnin-Viggars, O, Daly, C, Dias, S, Stockton, S, Meiser-Stedman, R, et al. Research review: psychological and psychosocial treatments for children and young people with post-traumatic stress disorder: a network meta-analysis. J Child Psychol Psychiatry. (2020) 61:18–29. doi: 10.1111/jcpp.13094
CrossRef Full Text | Google Scholar
171. Mitchell, KS, Mazzeo, SE, Rausch, SM, and Cooke, KL. Innovative interventions for disordered eating: evaluating dissonance-based and yoga interventions. Int J Eat Disord. (2007) 40:120–8. doi: 10.1002/eat.20282
PubMed Abstract | CrossRef Full Text | Google Scholar
172. Seppala, EM, Nitschke, JB, Tudorascu, DL, Hayes, A, Goldstein, MR, Nguyen, DT, et al. Breathing-based meditation decreases posttraumatic stress disorder symptoms in U.S. military veterans: a randomized controlled longitudinal study. J Trauma Stress. (2014) 27:397–405. doi: 10.1002/jts.21936
PubMed Abstract | CrossRef Full Text | Google Scholar
173. Iverson, KM, Litwack, SD, Pineles, SL, Suvak, MK, Vaughn, RA, and Resick, PA. Predictors of intimate partner violence revictimization: the relative impact of distinct PTSD symptoms, dissociation, and coping strategies. J Trauma Stress. (2013) 26:102–10. doi: 10.1002/jts.21781
PubMed Abstract | CrossRef Full Text | Google Scholar
174. Bajor, LA, Balsara, C, and Osser, DN. An evidence-based approach to psychopharmacology for posttraumatic stress disorder (PTSD) -2022 update. Psychiatry Res. (2022) 317:114840. doi: 10.1016/j.psychres.2022.114840
PubMed Abstract | CrossRef Full Text | Google Scholar
176. Himmerich, H, Kan, C, Au, K, and Treasure, J. Pharmacological treatment of eating disorders, comorbid mental health problems, malnutrition and physical health consequences. Pharmacol Ther. (2021) 217:107667. doi: 10.1016/j.pharmthera.2020.107667
PubMed Abstract | CrossRef Full Text | Google Scholar
177. Himmerich, H, and Treasure, J. Psychopharmacological advances in eating disorders. Expert Rev Clin Pharmacol. (2018) 11:95–108. doi: 10.1080/17512433.2018.1383895
CrossRef Full Text | Google Scholar
178. Hoskins, MD, Bridges, J, Sinnerton, R, Nakamura, A, Underwood, JFG, Slater, A, et al. Pharmacological therapy for post-traumatic stress disorder: a systematic review and meta-analysis of monotherapy, augmentation and head-to-head approaches. Eur J Psychotraumatol. (2021) 12:1802920. doi: 10.1080/20008198.2020.1802920
CrossRef Full Text | Google Scholar
179. Hoskins, MD, Sinnerton, R, Nakamura, A, Underwood, JFG, Slater, A, Lewis, C, et al. Pharmacological-assisted psychotherapy for post-traumatic stress disorder: a systematic review and meta-analysis. Eur J Psychotraumatol. (2021) 12:1853379. doi: 10.1080/20008198.2020.1853379
CrossRef Full Text | Google Scholar
180. Aigner, M, Treasure, J, Kaye, W, Kasper, S, and Disorders, WTFOE. World Federation of Societies of biological psychiatry (WFSBP) guidelines for the pharmacological treatment of eating disorders. World J Biol Psychiatry. (2011) 12:400–43. doi: 10.3109/15622975.2011.602720
PubMed Abstract | CrossRef Full Text | Google Scholar
181. Amodeo, G, Cuomo, A, Bolognesi, S, Goracci, A, Trusso, MA, Piccinni, A, et al. Pharmacotherapeutic strategies for treating binge eating disorder. Evidence from clinical trials and implications for clinical practice. Expert Opin Pharmacother. (2019) 20:679–90. doi: 10.1080/14656566.2019.1571041
PubMed Abstract | CrossRef Full Text | Google Scholar
182. Arnold, LM, McElroy, SL, Hudson, JI, Welge, JA, Bennett, AJ, and Keck, PE. A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry. (2002) 63:1028–33. doi: 10.4088/JCP.v63n1113
PubMed Abstract | CrossRef Full Text | Google Scholar
183. Attia, E, Steinglass, JE, Walsh, BT, Wang, Y, Wu, P, Schreyer, C, et al. Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am J Psychiatry. (2019) 176:449–56. doi: 10.1176/appi.ajp.2018.18101125
PubMed Abstract | CrossRef Full Text | Google Scholar
184. Bajor, LA, Ticlea, AN, and Osser, DN. The psychopharmacology algorithm Project at the Harvard south shore program: an update on posttraumatic stress disorder. Harv Rev Psychiatry. (2011) 19:240–58. doi: 10.3109/10673229.2011.614483
PubMed Abstract | CrossRef Full Text | Google Scholar
185. Brewerton, TD. Antipsychotic agents in the treatment of anorexia nervosa: neuropsychopharmacologic rationale and evidence from controlled trials. Curr Psychiatry Rep. (2012) 14:398–405. doi: 10.1007/s11920-012-0287-6
PubMed Abstract | CrossRef Full Text | Google Scholar
186. Brownley, KA, Peat, CM, La Via, M, and Bulik, CM. Pharmacological approaches to the management of binge eating disorder. Drugs. (2015) 75:9–32. doi: 10.1007/s40265-014-0327-0
PubMed Abstract | CrossRef Full Text | Google Scholar
187. Carey, P, Suliman, S, Ganesan, K, Seedat, S, and Stein, DJ. Olanzapine monotherapy in posttraumatic stress disorder: efficacy in a randomized, double-blind, placebo-controlled study. Hum Psychopharmacol. (2012) 27:386–91. doi: 10.1002/hup.2238
PubMed Abstract | CrossRef Full Text | Google Scholar
188. Fluoxetine Bulimia Nervosa Collaborative Study Group. Fluoxetine in the treatment of bulimia nervosa. A multicenter, placebo-controlled, double-blind trial. Arch Gen Psychiatry. (1992) 49:139–47. doi: 10.1001/archpsyc.1992.01820020059008
PubMed Abstract | CrossRef Full Text | Google Scholar
189. Goldstein, DJ, Wilson, MG, Thompson, VL, Potvin, JH, and Rampey, AH. Long-term fluoxetine treatment of bulimia nervosa. Fluoxetine bulimia nervosa research group. Br J Psychiatry. (1995) 166:660–6. doi: 10.1192/bjp.166.5.660
PubMed Abstract | CrossRef Full Text | Google Scholar
190. Hay, PJ, and Claudino, AM. Clinical psychopharmacology of eating disorders: a research update. Int J Neuropsychopharmacol. (2012) 15:209–22. doi: 10.1017/S1461145711000460
CrossRef Full Text | Google Scholar
191. Hedges, DW, Reimherr, FW, Hoopes, SP, Rosenthal, NR, Kamin, M, Karim, R, et al. Treatment of bulimia nervosa with topiramate in a randomized, double-blind, placebo-controlled trial, part 2: improvement in psychiatric measures. J Clin Psychiatry. (2003) 64:1449–54. doi: 10.4088/JCP.v64n1208
PubMed Abstract | CrossRef Full Text | Google Scholar
192. Hoopes, SP, Reimherr, FW, Hedges, DW, Rosenthal, NR, Kamin, M, Karim, R, et al. Treatment of bulimia nervosa with topiramate in a randomized, double-blind, placebo-controlled trial, part 1: improvement in binge and purge measures. J Clin Psychiatry. (2003) 64:1335–41. doi: 10.4088/JCP.v64n1109
PubMed Abstract | CrossRef Full Text | Google Scholar
193. McElroy, SL, Arnold, LM, Shapira, NA, Keck, PE Jr, Rosenthal, NR, Karim, MR, et al. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatry. (2003) 160:255–61. doi: 10.1176/appi.ajp.160.2.255
PubMed Abstract | CrossRef Full Text | Google Scholar
194. Milano, W, De Rosa, M, Milano, L, Riccio, A, Sanseverino, B, and Capasso, A. The pharmacological options in the treatment of eating disorders. ISRN Pharmacol. (2013) 2013:352865. doi: 10.1155/2013/352865
PubMed Abstract | CrossRef Full Text | Google Scholar
195. Pae, CU, Lim, HK, Peindl, K, Ajwani, N, Serretti, A, Patkar, AA, et al. The atypical antipsychotics olanzapine and risperidone in the treatment of posttraumatic stress disorder: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Int Clin Psychopharmacol. (2008) 23:1–8. doi: 10.1097/YIC.0b013e32825ea324
PubMed Abstract | CrossRef Full Text | Google Scholar
196. Romano, SJ, Halmi, KA, Sarkar, NP, Koke, SC, and Lee, JS. A placebo-controlled study of fluoxetine in continued treatment of bulimia nervosa after successful acute fluoxetine treatment. Am J Psychiatry. (2002) 159:96–102. doi: 10.1176/appi.ajp.159.1.96
PubMed Abstract | CrossRef Full Text | Google Scholar
197. Stein, MB, Kline, NA, and Matloff, JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry. (2002) 159:1777–9. doi: 10.1176/appi.ajp.159.10.1777
PubMed Abstract | CrossRef Full Text | Google Scholar
199. Watts, BV, Schnurr, PP, Mayo, L, Young-Xu, Y, Weeks, WB, and Friedman, MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. (2013) 74:e541–50. doi: 10.4088/JCP.12r08225
CrossRef Full Text | Google Scholar
201. Carbone, EA, Caroleo, M, Rania, M, Calabro, G, Staltari, FA, de Filippis, R, et al. An open-label trial on the efficacy and tolerability of naltrexone/bupropion SR for treating altered eating behaviours and weight loss in binge eating disorder. Eat Weight Disord. (2020) 26:779–88. doi: 10.1007/s40519-020-00910-x
CrossRef Full Text | Google Scholar
202. Casner, JA, Weinheimer, B, and Gualtieri, CT. Naltrexone and self-injurious behavior: a retrospective population study. J Clin Psychopharmacol. (1996) 16:389–94. doi: 10.1097/00004714-199610000-00008
PubMed Abstract | CrossRef Full Text | Google Scholar
203. Foa, EB, Yusko, DA, McLean, CP, Suvak, MK, Bux, DA Jr, Oslin, D, et al. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA. (2013) 310:488–95. doi: 10.1001/jama.2013.8268
PubMed Abstract | CrossRef Full Text | Google Scholar
204. Godart, NT, Agman, G, Perdereau, F, and Jeammet, P. Naltrexone treatment of self-injurious behavior. J Am Acad Child Adolesc Psychiatry. (2000) 39:1076–8. doi: 10.1097/00004583-200009000-00005
CrossRef Full Text | Google Scholar
205. Leary, M, Pursey, KM, Verdejo-Garcia, A, and Burrows, TL. Current intervention treatments for food addiction: a systematic review. Behav Sci. (2021) 11:80. doi: 10.3390/bs11060080
PubMed Abstract | CrossRef Full Text | Google Scholar
206. Leroy, A, Carton, L, Gomajee, H, Bordet, R, and Cottencin, O. Naltrexone in the treatment of binge eating disorder in a patient with severe alcohol use disorder: a case report. Am J Drug Alcohol Abuse. (2017) 43:618–20. doi: 10.1080/00952990.2017.1298117
PubMed Abstract | CrossRef Full Text | Google Scholar
207. O’Malley, SS, Sinha, R, Grilo, CM, Capone, C, Farren, CK, McKee, SA, et al. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: a randomized controlled trial. Alcohol Clin Exp Res. (2007) 31:625–34. doi: 10.1111/j.1530-0277.2007.00347.x
PubMed Abstract | CrossRef Full Text | Google Scholar
208. Palpacuer, C, Duprez, R, Huneau, A, Locher, C, Boussageon, R, Laviolle, B, et al. Pharmacologically controlled drinking in the treatment of alcohol dependence or alcohol use disorders: a systematic review with direct and network meta-analyses on nalmefene, naltrexone, acamprosate, baclofen and topiramate. Addiction. (2018) 113:220–37. doi: 10.1111/add.13974
PubMed Abstract | CrossRef Full Text | Google Scholar
209. Ray, LA, Krull, JL, and Leggio, L. The effects of naltrexone among alcohol non-abstainers: results from the COMBINE study. Front Psychol. (2010) 1:26. doi: 10.3389/fpsyt.2010.00026
CrossRef Full Text | Google Scholar
210. Shorter, D, Hsieh, J, and Kosten, TR. Pharmacologic management of comorbid post-traumatic stress disorder and addictions. Am J Addict. (2015) 24:705–12. doi: 10.1111/ajad.12306
PubMed Abstract | CrossRef Full Text | Google Scholar
211. Karayol, R, Medrihan, L, Warner-Schmidt, JL, Fait, BW, Rao, MN, Holzner, EB, et al. Serotonin receptor 4 in the hippocampus modulates mood and anxiety. Mol Psychiatry. (2021) 26:2334–49. doi: 10.1038/s41380-020-00994-y
PubMed Abstract | CrossRef Full Text | Google Scholar
212. Ragnhildstveit, A, Slayton, M, Jackson, LK, Brendle, M, Ahuja, S, Holle, W, et al. Ketamine as a novel psychopharmacotherapy for eating disorders: evidence and future directions. Brain Sci. (2022) 12:382. doi: 10.3390/brainsci12030382
PubMed Abstract | CrossRef Full Text | Google Scholar
213. Needs, P, Mote, SD, Manocchia, M, King, JD, Szuba, DD, Lustig, SL, et al. Psychotherapy and psychopharmacology utilization following repetitive transcranial magnetic stimulation (rTMS) in patients with major depressive disorder. Psychiatry Res. (2019) 278:51–5. doi: 10.1016/j.psychres.2019.05.020
PubMed Abstract | CrossRef Full Text | Google Scholar
214. Woodside, DB, Colton, P, Lam, E, Dunlop, K, Rzeszutek, J, and Downar, J. Dorsomedial prefrontal cortex repetitive transcranial magnetic stimulation treatment of posttraumatic stress disorder in eating disorders: An open-label case series. Int J Eat Disord. (2017) 50:1231–4. doi: 10.1002/eat.22764
PubMed Abstract | CrossRef Full Text | Google Scholar
215. Murray, SB, Strober, M, Tadayonnejad, R, Bari, AA, and Feusner, JD. Neurosurgery and neuromodulation for anorexia nervosa in the 21st century: a systematic review of treatment outcomes. Eat Disord. (2020) 30:26–53. doi: 10.1080/10640266.2020.1790270
CrossRef Full Text | Google Scholar
216. Hermida, AP, Glass, OM, Shafi, H, and McDonald, WM. Electroconvulsive therapy in depression: current practice and future direction. Psychiatr Clin North Am. (2018) 41:341–53. doi: 10.1016/j.psc.2018.04.001
PubMed Abstract | CrossRef Full Text | Google Scholar
217. Abdallah, CG, Roache, JD, Averill, LA, Young-McCaughan, S, Martini, B, Gueorguieva, R, et al. Repeated ketamine infusions for antidepressant-resistant PTSD: methods of a multicenter, randomized, placebo-controlled clinical trial. Contemp Clin Trials. (2019) 81:11–8. doi: 10.1016/j.cct.2019.04.009
PubMed Abstract | CrossRef Full Text | Google Scholar
218. Araujo-de-Freitas, L, Santos-Lima, C, Mendonca-Filho, E, Vieira, F, Franca, R, Magnavita, G, et al. Neurocognitive aspects of ketamine and esketamine on subjects with treatment-resistant depression: a comparative, randomized and double-blind study. Psychiatry Res. (2021) 303:114058. doi: 10.1016/j.psychres.2021.114058
PubMed Abstract | CrossRef Full Text | Google Scholar
219. Brewerton, TD, Lafrance, A, and Mithoefer, MC. The potential use of N-methyl-3,4-methylenedioxyamphetamine (MDMA) assisted psychotherapy in the treatment of eating disorders comorbid with PTSD. Med Hypotheses. (2021) 146:110367. doi: 10.1016/j.mehy.2020.110367
PubMed Abstract | CrossRef Full Text | Google Scholar
220. Calabrese, L, Scolnick, B, Zupec-Kania, B, Beckwith, C, Costello, K, and Frank, GKW. Ketogenic diet and ketamine infusion treatment to target chronic persistent eating disorder psychopathology in anorexia nervosa: a pilot study. Eat Weight Disord. (2022) 27:3751–7. doi: 10.1007/s40519-022-01455-x
PubMed Abstract | CrossRef Full Text | Google Scholar
221. Feder, A, Parides, MK, Murrough, JW, Perez, AM, Morgan, JE, Saxena, S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. (2014) 71:681–8. doi: 10.1001/jamapsychiatry.2014.62
PubMed Abstract | CrossRef Full Text | Google Scholar
222. Horn, SR, Charney, DS, and Feder, A. Understanding resilience: new approaches for preventing and treating PTSD. Exp Neurol. (2016) 284:119–32. doi: 10.1016/j.expneurol.2016.07.002
PubMed Abstract | CrossRef Full Text | Google Scholar
223. Jerome, L, Feduccia, AA, Wang, JB, Hamilton, S, Yazar-Klosinski, B, Emerson, A, et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacology. (2020) 237:2485–97. doi: 10.1007/s00213-020-05548-2
PubMed Abstract | CrossRef Full Text | Google Scholar
224. Kelmendi, B, Kichuk, SA, DePalmer, G, Maloney, G, Ching, THW, Belser, A, et al. Single-dose psilocybin for treatment-resistant obsessive-compulsive disorder: a case report. Heliyon. (2022) 8:e12135. doi: 10.1016/j.heliyon.2022.e12135
PubMed Abstract | CrossRef Full Text | Google Scholar
225. Knatz Peck, S, Shao, S, Murray, S, and Kaye, W. P450. Pilot study evaluation of psilocybin therapy for anorexia nervosa: safety, acceptability, and preliminary efficacy. Biol Psychiatry. (2022) 91:S270. doi: 10.1016/j.biopsych.2022.02.686
CrossRef Full Text | Google Scholar
226. Lafrance, A, Loizaga-Velder, A, Fletcher, J, Renelli, M, Files, N, and Tupper, KW. Nourishing the Spirit: exploratory research on Ayahuasca experiences along the continuum of recovery from eating Disorders. J Psychoactive Drugs. (2017) 49:427–35. doi: 10.1080/02791072.2017.1361559
PubMed Abstract | CrossRef Full Text | Google Scholar
227. Mithoefer, MC, Grob, CS, and Brewerton, TD. Novel psychopharmacological therapies for psychiatric disorders: psilocybin and MDMA. Lancet Psychiatry. (2016) 3:481–8. doi: 10.1016/S2215-0366(15)00576-3
PubMed Abstract | CrossRef Full Text | Google Scholar
228. Mohamed, A, Touheed, S, Ahmed, M, Hor, M, and Fatima, S. The efficacy of psychedelic-assisted therapy in managing post-traumatic stress disorder (PTSD): a new frontier? Cureus. (2022) 14:e30919. doi: 10.7759/cureus.30919
PubMed Abstract | CrossRef Full Text | Google Scholar
229. Nicholas, CR, Wang, JB, Coker, A, Mitchell, JM, Klaire, SS, Yazar-Klosinski, B, et al. The effects of MDMA-assisted therapy on alcohol and substance use in a phase 3 trial for treatment of severe PTSD. Drug Alcohol Depend. (2022) 233:109356. doi: 10.1016/j.drugalcdep.2022.109356
PubMed Abstract | CrossRef Full Text | Google Scholar
230. Nygart, VA, Pommerencke, LM, Haijen, E, Kettner, H, Kaelen, M, Mortensen, EL, et al. Antidepressant effects of a psychedelic experience in a large prospective naturalistic sample. J Psychopharmacol. (2022) 36:932–42. doi: 10.1177/02698811221101061
PubMed Abstract | CrossRef Full Text | Google Scholar
231. Reiff, CM, Richman, EE, Nemeroff, CB, Carpenter, LL, Widge, AS, Rodriguez, CI, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. (2020) 177:391–410. doi: 10.1176/appi.ajp.2019.19010035
PubMed Abstract | CrossRef Full Text | Google Scholar
232. Dandekar, MP, Fenoy, AJ, Carvalho, AF, Soares, JC, and Quevedo, J. Deep brain stimulation for treatment-resistant depression: an integrative review of preclinical and clinical findings and translational implications. Mol Psychiatry. (2018) 23:1094–112. doi: 10.1038/mp.2018.2
PubMed Abstract | CrossRef Full Text | Google Scholar
233. Kisely, S, Li, A, Warren, N, and Siskind, D. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. (2018) 35:468–80. doi: 10.1002/da.22746
PubMed Abstract | CrossRef Full Text | Google Scholar
234. Mayberg, HS, Lozano, AM, Voon, V, McNeely, HE, Seminowicz, D, Hamani, C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. (2005) 45:651–60. doi: 10.1016/j.neuron.2005.02.014
PubMed Abstract | CrossRef Full Text | Google Scholar
235. Sobstyl, M, Stapinska-Syniec, A, Sokol-Szawlowska, M, and Kupryjaniuk, A. Deep brain stimulation for the treatment of severe intractable anorexia nervosa. Br J Neurosurg. (2019) 33:601–7. doi: 10.1080/02688697.2019.1667484
PubMed Abstract | CrossRef Full Text | Google Scholar
236. Monteleone, AM, and Cascino, G. A systematic review of network analysis studies in eating disorders: is time to broaden the core psychopathology to non specific symptoms. Eur Eat Disord Rev. (2021) 29:531–47. doi: 10.1002/erv.2834
PubMed Abstract | CrossRef Full Text | Google Scholar
237. Smith, KE, Mason, TB, Crosby, RD, Cao, L, Leonard, RC, Wetterneck, CT, et al. A comparative network analysis of eating disorder psychopathology and co-occurring depression and anxiety symptoms before and after treatment. Psychol Med. (2019) 49:314–24. doi: 10.1017/S0033291718000867
PubMed Abstract | CrossRef Full Text | Google Scholar
238. Solmi, M, Collantoni, E, Meneguzzo, P, Degortes, D, Tenconi, E, and Favaro, A. Network analysis of specific psychopathology and psychiatric symptoms in patients with eating disorders. Int J Eat Disord. (2018) 51:680–92. doi: 10.1002/eat.22884
PubMed Abstract | CrossRef Full Text | Google Scholar
239. Ro, O, Martinsen, EW, Hoffart, A, Sexton, H, and Rosenvinge, JH. The interaction of personality disorders and eating disorders: a two-year prospective study of patients with longstanding eating disorders. Int J Eat Disord. (2005) 38:106–11. doi: 10.1002/eat.20166
PubMed Abstract | CrossRef Full Text | Google Scholar
240. Vrabel, KR, Ro, O, Martinsen, EW, Hoffart, A, and Rosenvinge, JH. Five-year prospective study of personality disorders in adults with longstanding eating disorders. Int J Eat Disord. (2010) 43:22–8. doi: 10.1002/eat.20662
PubMed Abstract | CrossRef Full Text | Google Scholar
241. Horigian, VE, Weems, CF, Robbins, MS, Feaster, DJ, Ucha, J, Miller, M, et al. Reductions in anxiety and depression symptoms in youth receiving substance use treatment. Am J Addict. (2013) 22:329–37. doi: 10.1111/j.1521-0391.2013.12031.x
PubMed Abstract | CrossRef Full Text | Google Scholar
243. Foa, EB, Rothbaum, BO, Riggs, DS, and Murdock, TB. Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive-behavioral procedures and counseling. J Consult Clin Psychol. (1991) 59:715–23. doi: 10.1037/0022-006X.59.5.715
PubMed Abstract | CrossRef Full Text | Google Scholar
244. Resick, PA, Williams, LF, Suvak, MK, Monson, CM, and Gradus, JL. Long-term outcomes of cognitive-behavioral treatments for posttraumatic stress disorder among female rape survivors. J Consult Clin Psychol. (2012) 80:201–10. doi: 10.1037/a0026602
PubMed Abstract | CrossRef Full Text | Google Scholar
245. Schnurr, PP, Friedman, MJ, Engel, CC, Foa, EB, Shea, MT, Chow, BK, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. (2007) 297:820–30. doi: 10.1001/jama.297.8.820
PubMed Abstract | CrossRef Full Text | Google Scholar
246. Batelaan, NM, Bosman, RC, Muntingh, A, Scholten, WD, Huijbregts, KM, and van Balkom, A. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. (2017) 358:3927. doi: 10.1136/bmj.j3927
CrossRef Full Text | Google Scholar
247. Carter, JC, McFarlane, TL, Bewell, C, Olmsted, MP, Woodside, DB, Kaplan, AS, et al. Maintenance treatment for anorexia nervosa: a comparison of cognitive behavior therapy and treatment as usual. Int J Eat Disord. (2009) 42:202–7. doi: 10.1002/eat.20591
PubMed Abstract | CrossRef Full Text | Google Scholar
248. DeRubeis, RJ, Zajecka, J, Shelton, RC, Amsterdam, JD, Fawcett, J, Xu, C, et al. Prevention of recurrence after recovery from a major depressive episode with antidepressant medication alone or in combination with cognitive behavioral therapy phase 2 of a 2-phase randomized clinical trial. JAMA Psychiatry. (2020) 77:237–45. doi: 10.1001/jamapsychiatry.2019.3900
PubMed Abstract | CrossRef Full Text | Google Scholar
249. Drach, LL, Maher, JE, Braun, MJ, Murray, SL, and Sazie, E. Substance use, disordered eating, and weight gain: describing the prevention and treatment Needs of incarcerated women. J Correct Health Care. (2016) 22:139–45. doi: 10.1177/1078345816634692
PubMed Abstract | CrossRef Full Text | Google Scholar
250. Krijnen-de Bruin, E, Scholten, W, Muntingh, A, Maarsingh, O, van Meijel, B, van Straten, A, et al. Psychological interventions to prevent relapse in anxiety and depression: a systematic review and meta-analysis. PLoS One. (2022) 17:e0272200. doi: 10.1371/journal.pone.0272200
PubMed Abstract | CrossRef Full Text | Google Scholar
251. Marlatt, GA, and Donovan, DM. Relapse prevention: mainenance strategies in the treatment of addictive behaviors, New York, NY: Guilford Press (2005).
Google Scholar
252. Pike, KM, Walsh, BT, Vitousek, K, Wilson, GT, and Bauer, J. Cognitive behavior therapy in the posthospitalization treatment of anorexia nervosa. Am J Psychiatry. (2003) 160:2046–9. doi: 10.1176/appi.ajp.160.11.2046
PubMed Abstract | CrossRef Full Text | Google Scholar
253. Vrana, C, Killeen, T, Brant, V, Mastrogiovanni, J, and Baker, NL. Rationale, design, and implementation of a clinical trial of a mindfulness-based relapse prevention protocol for the treatment of women with comorbid post traumatic stress disorder and substance use disorder. Contemp Clin Trials. (2017) 61:108–14. doi: 10.1016/j.cct.2017.07.024
PubMed Abstract | CrossRef Full Text | Google Scholar
254. Walsh, BT, Kaplan, AS, Attia, E, Olmsted, M, Parides, M, Carter, JC, et al. Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA. (2006) 295:2605–12. doi: 10.1001/jama.295.22.2605
PubMed Abstract | CrossRef Full Text | Google Scholar
255. Zhang, Z, Zhang, L, Zhang, G, Jin, J, and Zheng, Z. The effect of CBT and its modifications for relapse prevention in major depressive disorder: a systematic review and meta-analysis. BMC Psychiatry. (2018) 18:50. doi: 10.1186/s12888-018-1610-5
CrossRef Full Text | Google Scholar
256. Berends, T, Boonstra, N, and van Elburg, A. Relapse in anorexia nervosa: a systematic review and meta-analysis. Curr Opin Psychiatry. (2018) 31:445–55. doi: 10.1097/YCO.0000000000000453
PubMed Abstract | CrossRef Full Text | Google Scholar
257. Khalsa, SS, Portnoff, LC, McCurdy-McKinnon, D, and Feusner, JD. What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. J Eat Disord. (2017) 5:20. doi: 10.1186/s40337-017-0145-3
CrossRef Full Text | Google Scholar
258. Walsh, BT, Xu, T, Wang, Y, Attia, E, and Kaplan, AS. Time course of relapse following acute treatment for anorexia nervosa. Am J Psychiatry. (2021) 178:848–53. doi: 10.1176/appi.ajp.2021.21010026
PubMed Abstract | CrossRef Full Text | Google Scholar
259. Brewerton, TD, Alexander, J, and Schaefer, J. Trauma-informed care and practice for eating disorders: personal and professional perspectives of lived experiences. Eat Weight Disord. (2019) 24:329–38. doi: 10.1007/s40519-018-0628-5
PubMed Abstract | CrossRef Full Text | Google Scholar
260. Conti, J, Rhodes, P, and Adams, H. Listening in the dark: why we need stories of people living with severe and enduring anorexia nervosa. J Eat Disord. (2016) 4:33. doi: 10.1186/s40337-016-0117-z
CrossRef Full Text | Google Scholar
261. Dawson, L, Mullan, B, Touyz, S, and Rhodes, P. Are recovery stories helpful for women with eating disorders? A pilot study and commentary on future research. J Eat Disord. (2018) 6:21. doi: 10.1186/s40337-018-0206-2
CrossRef Full Text | Google Scholar
262. Eddy, KT, Tabri, N, Thomas, JJ, Murray, HB, Keshaviah, A, Hastings, E, et al. Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. J Clin Psychiatry. (2017) 78:184–9. doi: 10.4088/JCP.15m10393
CrossRef Full Text | Google Scholar
263. Elwyn, R. A lived experience response to the proposed diagnosis of terminal anorexia nervosa: learning from iatrogenic harm, ambivalence and enduring hope. J Eat Disord. (2023) 11:2. doi: 10.1186/s40337-022-00729-0
CrossRef Full Text | Google Scholar
264. Franko, DL, Tabri, N, Keshaviah, A, Murray, HB, Herzog, DB, Thomas, JJ, et al. Predictors of long-term recovery in anorexia nervosa and bulimia nervosa: data from a 22-year longitudinal study. J Psychiatr Res. (2018) 96:183–8. doi: 10.1016/j.jpsychires.2017.10.008
PubMed Abstract | CrossRef Full Text | Google Scholar
265. Kotilahti, E, West, M, Isomaa, R, Karhunen, L, Rocks, T, and Ruusunen, A. Treatment interventions for severe and enduring eating Disorders: systematic review. Int J Eat Disord. (2020) 53:1280–302. doi: 10.1002/eat.23322
PubMed Abstract | CrossRef Full Text | Google Scholar
266. Music, S, Elwyn, R, Fountas, G, Gnatt, I, Jenkins, ZM, Malcolm, A, et al. Valuing the voice of lived experience of eating disorders in the research process: benefits and considerations. Aust N Z J Psychiatry. (2021) 56:216–8. doi: 10.1177/0004867421998794
CrossRef Full Text | Google Scholar
267. Olofsson, ME, Oddli, HW, Vrabel, KAR, and Hoffart, A. «In solitude is safeness»: a patient perspective on eating disorders in the context of multiple childhood trauma. Nordic Psychol. (2020) 73:29–42. doi: 10.1080/19012276.2020.1762714
CrossRef Full Text | Google Scholar
268. Pinar-Gutierrez, A, Dios-Fuentes, E, Remon-Ruiz, P, Del Can-Sanchez, D, Vazquez-Morejon, A, Lopez-Narbona, M, et al. Description of characteristics and outcomes of a cohort of patients with severe and enduring eating disorders (SE-ED). J Eat Disord. (2021) 9:135. doi: 10.1186/s40337-021-00492-8
CrossRef Full Text | Google Scholar
269. Roer, GE, Solbakken, HH, Abebe, DS, Aaseth, JO, Bolstad, I, and Lien, L. Inpatients experiences about the impact of traumatic stress on eating behaviors: an exploratory focus group study. J Eat Disord. (2021) 9:119. doi: 10.1186/s40337-021-00480-y
CrossRef Full Text | Google Scholar
270. Wonderlich, SA, Bulik, CM, Schmidt, U, Steiger, H, and Hoek, HW. Severe and enduring anorexia nervosa: update and observations about the current clinical reality. Int J Eat Disord. (2020) 53:1303–12. doi: 10.1002/eat.23283
PubMed Abstract | CrossRef Full Text | Google Scholar
271. Guarda, AS, Hanson, A, Mehler, P, and Westmoreland, P. Terminal anorexia nervosa is a dangerous term: it cannot, and should not, be defined. J Eat Disord. (2022) 10:79. doi: 10.1186/s40337-022-00599-6
CrossRef Full Text | Google Scholar
272. Mack, RA, and Stanton, CE. Responding to “terminal anorexia nervosa: three cases and proposed clinical characteristics”. J Eat Disord. (2022) 10:87. doi: 10.1186/s40337-022-00612-y
CrossRef Full Text | Google Scholar
273. Riddle, M, O’Melia, AM, and Bauschka, M. First, do no harm: the proposed definition of “terminal anorexia” is fraught with danger for vulnerable individuals. J Eat Disord. (2022) 10:81. doi: 10.1186/s40337-022-00605-x
CrossRef Full Text | Google Scholar
274. Westmoreland, P, and Mehler, PS. Caring for patients with severe and enduring eating disorders (SEED): certification, harm reduction, palliative care, and the question of futility. J Psychiatr Pract. (2016) 22:313–20. doi: 10.1097/PRA.0000000000000160
PubMed Abstract | CrossRef Full Text | Google Scholar