Supplemental material accompanies this paper
Subjects
Male Wistar rats (n = 134; Charles River, Wilmington, MA) were single-housed in a 12-h reverse light cycle (lights off at 11:00 a.m.) in an AAALAC-approved humidity-controlled and temperature-controlled vivarium. Rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012; 44% kcal carbohydrate, 5.8% fat, 19% protein, metabolizable energy 310 cal/100 g; Harlan, Indianapolis, IN) and free access to water at all times with the exception of certain experimental test procedures. Procedures used in this study adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee (approval no. 15174). Each experiment used an independent cohort of animals, with the exception of CPP, which took place prior to ICSS experiments.
Drugs
d-Amphetamine (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% sterile saline for intraperitoneal (i.p.) injections.
Ad libitum alternation of a standard chow with palatable diet
Ad libitum alternation of a standard chow with palatable diet was performed as described previously [4, 17, 24, 25] and continued for ≥5 weeks preceding all experiments. Briefly, control rats were provided with free access to a chow diet 7 days a week (Chow/Chow) and experimental rats were provided with free access to chow for 5 days a week, followed by 2 days of free access to a palatable diet (Chow/Palatable). The “chow” diet was the above-described corn-based chow, and the palatable diet was a nutritionally complete, chocolate-flavored, high-sucrose (50% kcal), AIN-76A-based diet that is comparable in macronutrient proportions and energy density to the chow diet (45 mg, 5TUL: 66.7% kcal carbohydrate, 12.7% fat, 20.6% protein, metabolizable energy 344 kcal/100 g; TestDiet, Richmond, IN). For brevity, the first 5 days (chow only) and the last 2 days (chow or palatable diet according to the experimental group) of each week are referred to in all experiments as C and P Phase, respectively. Diets were never concurrently available.
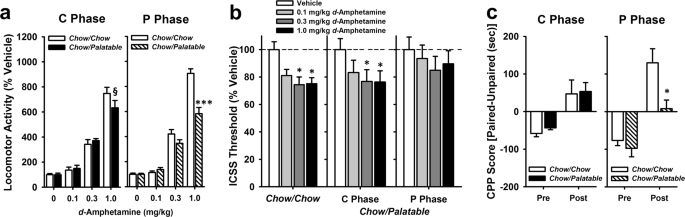
Locomotor activity
A locomotor activity assay was used to test sensitivity to the stimulating effects of d-Amphetamine. One day after food switch, rats (Chow/Chow, n = 12; Chow/Palatable, n = 12) were injected with saline or d-Amphetamine (0.1, 0.3, and 1 mg/kg, i.p.) using a counterbalanced, within-subject, Latin square design. All rats received each dose during both the C and P Phase, >5 days apart, and were compared against the Chow/Chow rats run on the same day. Locomotor response to d-Amphetamine is operationalized as the increase in locomotor activity as compared to locomotor activity under vehicle baseline conditions.
Intracranial self-stimulation (ICSS)
An ICSS procedure was used to assess how d-Amphetamine potentiates brain stimulation reward (BSR) (i.e. lowers the BSR threshold), a measure of reward system functioning). BSR thresholds were determined using the rate-independent discrete-trial current intensity procedure [26, 27]. In each session, rats were required to lever press for electrical stimulation at varying current intensities. There were six total sets of trials (termed “columns”) where current intensities were varied in either ascending or descending patterns. Within each column, rats performed five trials at each current intensity: ≥3 out of 5 responses was considered a positive response at that intensity, while ≤2 responses was considered a negative response. The threshold (i.e. current intensity where self-stimulation behavior ceases, defined as the midpoint between the last “positive response” intensity and the first “negative response” intensity) was determined for each column and averaged over the last four columns of each session. A decrease in the reward threshold reflects an increase in reward function as measured by ICSS [26, 28, 29]. On test days, rats were administered saline or d-Amphetamine (0.1, 0.3, 1.0 mg/kg, i.p.) immediately prior to the session start (one day post-diet switch), in a counterbalanced, within-subject, Latin square design (final group sizes: Chow/Chow, n = 7; Chow/Palatable, n = 16).
Conditioned place preference (CPP)
A biased CPP procedure was used to test the rewarding effects of d-Amphetamine in diet alternated rats. Following 6 weeks of diet alternation, Chow/Palatable rats were maintained on either the chow food (C Phase group) or the palatable food (P Phase group) for the duration of CPP training/testing (7 days) and each group was run simultaneously with a Chow/Chow control group. During preconditioning, rats were allowed to freely explore the apparatus for 15 min. On alternate days, rats (C Phase testing: Chow/Chow, n = 8, Chow/Palatable: C Phase, n = 13; P Phase testing: Chow/Chow, n = 6, Chow/Palatable: P Phase, n = 12) were given an injection of saline or d-Amphetamine (1.0 mg/kg, i.p.) and confined to one chamber for 25 min, three pairings each (d-Amphetamine = unbiased chamber; saline = biased chamber). The day after the last conditioning session, rats were tested following the same procedure as the preconditioning test. A CPP score was calculated (time in d-Amphetamine paired chamber–time in unpaired chamber [30, 31]).
In vivo microdialysis
An in vivo microdialysis procedure was used to determine NAc shell DA efflux in response to d-Amphetamine. Rats were stereotaxically implanted with a unilateral, intracranial cannula in the NAc shell, as described previously [32,33,34]. In vivo microdialysis occurred in rats one day post-diet switch (either C to P, or P to C Phase) for Chow/Palatable rats with Chow/Chow controls run simultaneously. Samples were collected at 10 min intervals at baseline (30 min), after saline injection (i.p.; 30 min), and after injection with d-Amphetamine (1 mg/kg, i.p.; 2 h). Samples were frozen and stored at -80 °C until high performance liquid chromatography (HPLC) analysis (final n sizes: Chow/Chow, n = 6; Chow/Palatable: C Phase, n = 8; Chow/Palatable: P Phase, n = 9).
Quantitative “no-net-flux” microdialysis
In order to determine baseline extracellular DA and DAT function, quantitative “no-net-flux” microdialysis methods were used. Methods were identical to in vivo microdialysis methods described above, unless otherwise noted. Quantitative “no-net-flux” microdialysis was performed in rats (Chow/Chow, n = 8; Chow/Palatable: C Phase, n = 7; Chow/Palatable: P Phase, n = 10) one day post-diet switch (either C to P or P to C Phase) for Chow/Palatable rats, with Chow/Chow controls run simultaneously. Varying concentrations of DA (0, 2.5, 5, and 10 nM) were added to the perfusate (1:3 mixture of aCSF and antioxidant, pH 6.8). A 90-min equilibration period was allowed after changing perfusate. Samples were collected at 10-min intervals for 30 min. Loss or gain of DA into the perfusate was calculated [DA concentration in perfusate−DA concentration in collected sample] and plotted in a regression against DA concentration in perfusate. The slope (extraction fraction) and x-intercept were then calculated using linear regression for each animal.
High-performance liquid chromatography (HPLC)
DA was measured by HPLC (HTEC-510, WE-3G electrochemical detector, Amuza Inc., San Diego, CA) [35, 36]. DA concentrations were determined by using standard curves with known amounts of DA in a range of 0.5–20 pg, with a detection limit of 0.02 pg. Retention times for DA were verified daily using standard solutions.
Histology
Rats that underwent in vivo microdialysis were sacrificed for brain histology placement at the culmination of the experiment.
Quantitative polymerase chain reaction (qPCR)
For the quantification of TH and DAT mRNA in the VTA, a separate, untested cohort of rats was sacrificed 24 h post-diet switch either from chow to palatable (P Phase, n = 8) or from palatable to chow (C Phase, n = 9), along with Chow/Chow controls (n = 6). Procedures were performed as described previously [37]. Results were analyzed by second derivative methods and expressed in arbitrary units (normalized to the reference gene Cyclophilin A, Cyp; (see Supplemental Table 1 for primers and conditions).
Statistical analysis
Data from locomotor activity, ICSS, CPP, PCR, and quantitative no-net-flux (slope and x-intercept) experiments, as well as body weights, were analyzed with simple or factorial ANOVAs. To control for unspecified day effects, locomotor activity and CPP were analyzed against the simultaneously run control group. In vivo microdialysis data were analyzed using linear mixed effects modeling in order to account for missing at random data.
Pairwise post hoc comparisons were made using Newman–Keuls after significant ANOVAs. For mixed linear effects analysis, we used Bonferroni correction, a more conservative post hoc. Partial eta squared is reported as effect size for ANOVAs (main effects and interactions), while Cohen’s d was calculated according to Cohen [38] in the event of significant differences between groups as determined by post hoc analysis.
The software/graphic packages used were SigmaPlot 12.0 (Systat Software Inc., Chicago, IL), SPSS Statistics 24 (IBM Corp., Armonk, NY), and Statistica 7.0 (StatSoft, Tulsa, OK).