Mice exhibit HFD overconsumption at 24 h post-PSS exposure
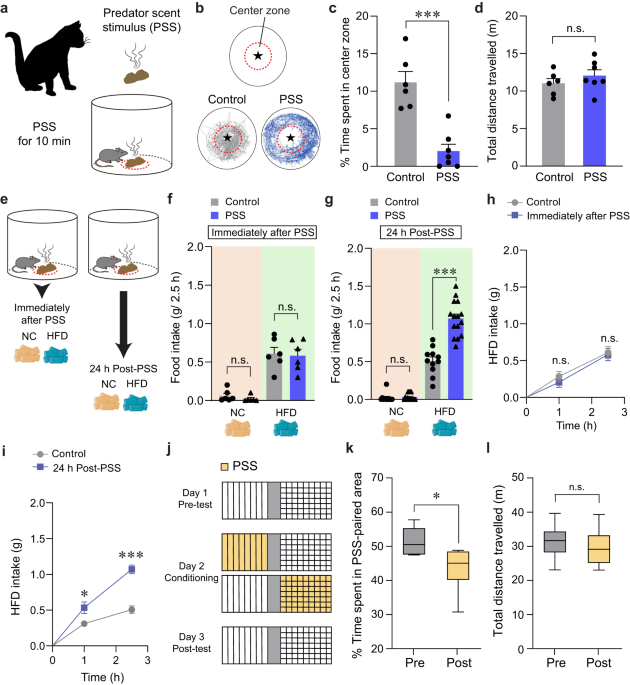
To understand how threatening stimuli trigger emotional stress responses, we used a PSS paradigm26,27 in which mice (8–10 weeks old) are exposed to a volatile predator cue (e.g., cat odor) that signifies imminent danger (Fig. 1a). To assess the acute impact of PSS on the development of negative emotions such as aversion, we exposed individual mice to a confined chamber with a PSS zone (center) and a non-PSS zone (periphery). We found that, in the presence of PSS, mice spend significantly more time in the periphery, maximizing their distance from the center with no accompanying changes in locomotion (Fig. 1b–d). This suggests that mice develop aversive emotional responses that prompt avoidance behaviors upon encountering PSS.
Fig. 1: Mice show enhanced food intake for HFD, but not NC, 24 h after PSS exposure.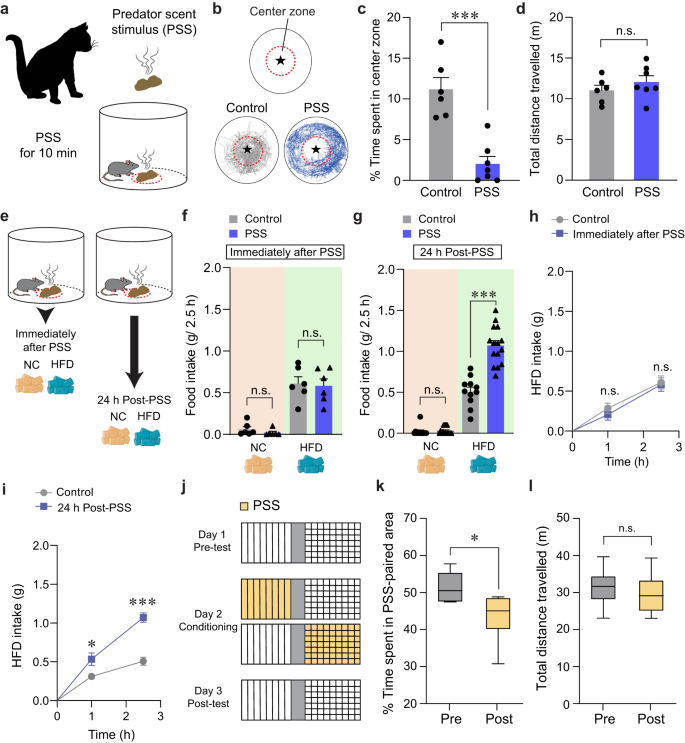
a Schematics of PSS exposure. b Asterisk denotes the location of the small wire mesh cage (top). Representative movement tracks in the presence of control or PSS (bottom). c, d Time spent (%) in the center zone and total distance traveled (n = 6, 7 mice per group). In (c) two-tailed unpaired t test, t11 = 5.491, ***p = 0.000189; In (d) two-tailed unpaired t test, t11 = −1.013, p = 0.333. e Schematic illustrating food consumption test after PSS. f 2.5 h food consumption immediately after PSS (n = 6 mice per group). In NC intake, two-tailed unpaired t test, t10 = 1.154, p = 0.275; In HFD intake, two-tailed unpaired t test, t10 = 0.217, p = 0.832. g 2.5 h food consumption 24 h after PSS (n = 11, 15 mice per group). In NC intake, two-tailed unpaired t test, t24 = −0.442, p = 0.663; In HFD intake, two-tailed unpaired t test, t24 = −6.693, ***p = 0.000000636. h, i Cumulative HFD intake immediately (h; n = 6 mice per group) or 24 h (i; n = 6 mice per group) after PSS exposure. In (h) two-way repeated-measures (RM) ANOVA (F(1,10) = 0.292, p = 0.601) was followed by Bonferroni post hoc test for multiple comparisons; p = 0.466, p = 0.814 compared with control mice; In (i) two-way RM ANOVA (F(1,10) = 18.390, p = 0.002) was followed by Bonferroni post hoc test for multiple comparisons; *p = 0.035, ***p j Experimental procedure to test CPA: one side of a two-sided chamber is paired with PSS on day 2. k, l Time spent (%) in PSS-paired chambers and total distance traveled (n = 6 mice). Box–whisker plots display median (center) and 2.5–97.5 percentiles of the distribution (bounds) with whiskers extending from min to max values. In (k) two-tailed paired t test, t5 = 3.776, *p = 0.0129; In (l) two-tailed paired t test, t5 = 0.719, p = 0.504. n.s not significant. Data are expressed as mean ± SEM.
Maladaptive eating behaviors, including the emotional overeating of palatable foods, are often considered coping mechanisms for individuals who have been primed with negative emotions after life-threatening events3,28. Given that the cellular or circuit-level changes underlying the stress-induced maladaptive behaviors require time to take hold29,30, there is likely a delay between PSS exposure and the onset of emotional eating. To determine whether such a delay is necessary for mice to develop emotional overeating after PSS, we subjected mice to HFD (60% kcal% fat) either immediately or 24 h after PSS exposure (Fig. 1e). Immediately after PSS exposure, we did not observe any changes in the HFD consumption regardless of PSS history. In contrast, 24 h after PSS exposure, the PSS mice consumed much more HFD in 2.5 h than mice that were never exposed to PSS (Fig. 1f–i). We did not see any PSS-dependent differences in normal chow (NC) intake among various groups of mice (Fig. 1f, g). These data suggest PSS enhances preference for palatable food in mice, particularly 24 h after exposure.
Emotional eating is triggered by negative emotions that arise and persist after stressful events. We thus asked whether PSS induces negative emotional valence in addition to HFD overconsumption at 24 h post-exposure. Using a classical conditioning paradigm, we found that mice exhibited a conditioned place avoidance (CPA) of cues previously paired with PSS and that this result is independent of any changes in locomotor activity (Fig. 1j–l). This suggests mice tend to retain negative emotions at 24 h post-PSS exposure. Together, these data support the hypothesis that PSS induces a delayed behavioral response that comprises a tendency to overconsume HFD accompanied by negative emotional valence.
Anatomical identification of LHPenk neurons
Exposure to stressful situations can promote palatable food consumption via increasing the hypothalamic circuit activity involved in eating behaviors5,6. To determine whether PSS promotes emotional eating by increasing hypothalamic activity in response to HFD, we quantified c-fos expression in several hypothalamic subregions after PSS exposure. We found that, both in the LH and PVN, mice exposed to PSS a day before showed remarkably enhanced c-fos expression in response to HFD compared to non-PSS controls (Supplementary Fig. 1a–d). While the dorsomedial hypothalamus (DMH) showed a modest increase in c-fos induction under the same condition, we did not detect significant changes in other hypothalamic subregions, such as the ventromedial hypothalamus (VMH) or Arc (Supplementary Fig. 1e–j). With our discovery that PSS mice overconsume HFD (Fig. 1g), these c-fos data suggest that the LH or PVN could mediate PSS-induced emotional overeating.
Penk, an endogenous opioid polypeptide hormone that produces enkephalin, has been implicated in several emotional behaviors, including fear conditioning, anxiety, and responses to stress17,31. Using Penk-Cre mice crossed with the Ai14 reporter mouse line, we found that Penk is highly expressed in the hypothalamic subregions, including the LH, PVN, and Arc (Fig. 2a, b). Because the LH is a neuroanatomical hub responsible for regulating diverse primitive behavioral states, such as feeding, as well as approach and avoidance behaviors7,12,13, we hypothesized that LHPenk neuronal population is likely to be responsible for PSS-induced emotional overeating. In the LH, however, there are other cell-type of subpopulations that are already known to be involved in controlling behavioral stress responses and feeding behaviors5,11,32, including pro-melanin concentrating hormone (Pmch)-, Hcrt-, and Lepr-expressing neurons. Therefore, we first sought to examine whether the LHPenk neurons are anatomically distinct from those subpopulations. Using a dual fluorescent in situ hybridization (FISH) experiment, we found only minor overlaps between LHPenk neurons and other cell-type of LH neurons expressing the Pmch, Hcrt, or Lepr, while the large proportions of LHPenk neurons (76–90%) represent a non-overlapping and discrete population (Fig. 2c–h and Supplementary Fig. 2a–c). Furthermore, using FISH to probe mRNA expression of VGAT and vGlut2, which are GABAergic and glutamatergic neuronal markers, respectively, we observed that LHPenk neurons are composed of subsets of inhibitory and excitatory cells (Fig. 2i, j and Supplementary Fig. 2d).
Fig. 2: The LH contains molecularly distinct Penk-expressing neurons.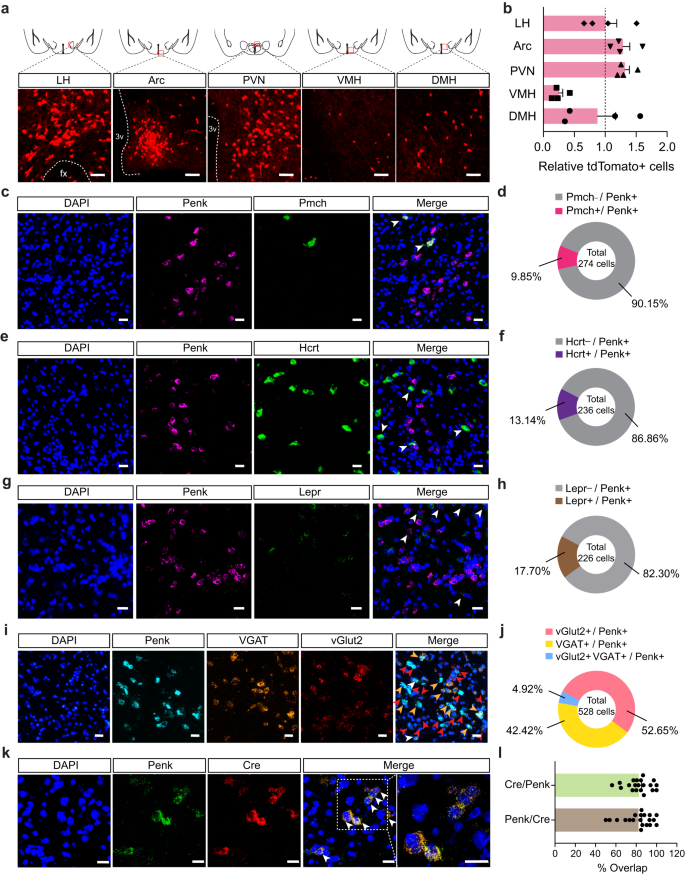
a Coronal diagrams depicting the region analyzed (squared in red; top) and confocal images showing the Penk expression in the multiple hypothalamic subregions of Penk-Cre × Ai14 mice (bottom), replicated independently with similar results in 4 mice. Scale bars, 50 μm. fx, fornix; 3 v, third ventricle. b Quantification of tdTomato-positive cells in the LH, Arc, PVN, VMH, and DMH (n = 4 Penk-Cre × Ai14 mice). Dashed line indicates the normalized level of tdTomato-positive cells in the LH. c–h, Representative images of RNA in situ hybridization for Pmch (c), Hcrt (e), and Lepr (g) with Penk in the LH. Arrowheads represent colocalization, replicated independently with similar results in 2 mice. Scale bars, 25 μm. Pie charts indicate % of LHPenk neurons colocalizing with Pmch (d; n = 274 cells from two mice), Hcrt (f; n = 236 cells from two mice), or Lepr (h; n = 226 cells from two mice). A large fraction of LHPenk neurons does not express Pmch, Hcrt, or Lepr. i, j Representative images of RNA in situ hybridization for VGAT and vGlut2 with Penk in the LH (i). Red, yellow, and white arrowheads represent colocalization of Penk with vGlut2, VGAT, and all respectively, replicated independently with similar results in 3 mice. Scale bars, 25 μm. Pie charts indicate % of LHPenk neurons colocalizing with vGlut2, VGAT and all (j; n = 528 cells from three mice). k, l Representative images of RNA in situ hybridization for Penk and Cre in the LH using Penk-Cre mice and its high magnification image (squared in white dashed line) (k). Arrowheads represent colocalization. Scale bars, 20 μm. Quantification of the % overlap of Cre+ cells as a fraction of Penk+ cells and Penk+ cells as a fraction of Cre+ cells (l). (n = 20 images). n.s not significant. Data are presented as mean ± SEM.
To gain a circuit-level understanding of how LHPenk neurons contribute to PSS-induced HFD overconsumption associated with negative emotional valence, we next aimed to delineate the input organization of the LHPenk circuitry. We injected Cre-dependent AAVs expressing TVA receptor and rabies virus glycoprotein (RVG) (AAV-DIO-mRuby2-TVA, AAV-DIO-RVG, respectively) into the LH of Penk-Cre mice validated to co-express Penk and Cre recombinase (Fig. 2k, l). We then delivered EnvA-pseudotyped, glycoprotein-deleted rabies virus (EnvA-RVΔG-eGFP) into the same area to map monosynaptic inputs to LHPenk neurons (Supplementary Fig. 3a–c). We found that LHPenk neurons receive synaptic inputs from the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), preoptic area (POA), PVN, central amygdala (CeA), and ventral tegmental area (VTA). Among these areas, the PVN -a principal regulator of the HPA axis33- is the primary input source of LHPenk neurons (Supplementary Fig. 3d, e). To investigate the molecular identity of the PVN input neurons, we combined virus-mediated input tracing with FISH and found that most of the PVN input neurons are glutamatergic (Supplementary Fig. 3f, g). Together, these data indicate that LHPenk neurons directly receive information from several brain areas that have been implicated in regulating central stress response systems or in the emotional/behavioral processing of aversive and rewarding stimuli33,34,35,36.
PSS enhances LHPenk neuronal responsiveness upon HFD consumption
Since the specific contributions of the LHPenk neurons to mediating PSS-induced emotional overeating remain unknown, we next asked how endogenous LHPenk neuronal activity is affected by PSS or HFD exposure. We investigated this by virally expressing Cre-dependent GCaMP6f in the LH of Penk-Cre mice and implanting an imaging cannula with a gradient index (GRIN) lens directly above the virus injection site (Fig. 3a, b). We then acquired single-cell-resolution in vivo Ca2+ imaging data from the GCaMP6f-expressing LHPenk neurons (Fig. 3c). To determine whether LHPenk neuronal activity is associated with PSS-induced aversive emotional responses, we monitored the dynamics of GCaMP6 fluorescence during a 10-min presentation of PSS. We found that when the mice were exposed to PSS, many LHPenk neurons showed an increase in the number of Ca2+ transients that then returned to baseline after PSS removal (Supplementary Fig. 4a–c). We also observed that more LHPenk neurons were activated (41.33%) than inhibited (16.00%) when PSS was presented (Supplementary Fig. 4d–g). In contrast, at the time of PSS removal, only 9.33% of LHPenk neurons were activated, but a relatively higher proportion of the cells (32.00%) was inhibited (Supplementary Fig. 4h–k). Notably, in the presence of an empty container with the same color and shape as the one we used for presenting the PSS, the LHPenk neurons exhibit no substantial differences in activity (Supplementary Fig. 4l–n). Together, these data suggest that LHPenk neuronal activity encodes the PSS-driven negative emotional state rather than general behavioral features evoked by novel stimuli.
Fig. 3: LHPenk neurons of PSS mice exhibit sensitized activity in response to HFD consumption.
a Schematic for the injection of AAV-DIO-GCaMP6f (left) and implantation of GRIN lens into the LH (right). b, c Confocal image showing GRIN lens placement on the GCaMP6f-expressing LHPenk neurons. Scale bar, 250 μm (b). A sample image for data acquisition. Scale bar, 50 μm (c), replicated independently with similar results in 4 mice. d Schematic for GRIN lens-implanted mice exposed to HFD. e, f Example in vivo Ca2+ activity traces from LHPenk neurons of control (e) and PSS mice (f) in response to the 1st HFD eating bout. g, h Mean z-score of the LHPenk neuronal activity of control (g; n = 55 cells from four control Penk-Cre mice) and PSS mice (h; n = 54 cells from five PSS Penk-Cre mice) before and after the 1st HFD eating bout. Two-tailed paired t test; in (g) t54 = −0.435, p = 0.665; in (h), t53 = −4.233, ***p = 0.0000921. i, j Pie charts indicate the classification of LHPenk neurons of control (i) and PSS mice (j) showing activated, inhibited, or unresponsive activity upon the 1st HFD eating bout. k, l Schematic for GRIN lens-implanted control (k) and PSS mice (l) in the presence of a novel object. In vivo Ca2+ activity is monitored in the presence of a novel object 24 h after PSS exposure. m, n As for (e, f), but in response to the 1st physical contact to a novel object. o, p Mean z-score of the LHPenk neuronal activity of control (o; n = 45 cells from four control Penk-Cre mice) and PSS mice (p; n = 56 cells from five PSS Penk-Cre mice) before and after the 1st physical contact to a novel object. Two-tailed paired t-test; in (o), t44 = −1.036, p = 0.306; in (p), t55 = −1.602, p = 0.115. q, r As for (i, j), but upon the 1st physical contact with a novel object. n.s not significant. Data are presented as mean ± SEM.
We next asked how PSS affects LHPenk neurons in the context of palatable food consumption. Based on our observation of emotional overeating 24 h after PSS exposure (Fig. 1g), we examined in vivo Ca2+ dynamics of PSS-primed LHPenk neurons in response to HFD eating (Fig. 3d). We found increased LHPenk neuronal activity in PSS mice upon the HFD eating onset, whereas non-PSS controls showed only a mild increase or no response under the same condition (Fig. 3e–h). Overall, we found that more than 48% of LHPenk neurons in PSS mice were activated upon the HFD eating onset compared to 30.91% of LHPenk neurons in control mice (Fig. 3i, j). In contrast to this, we found that novel objects evoked only subtle changes in LHPenk neuronal activity, regardless of previous PSS exposure (Fig. 3k–r). Together, these data indicate that PSS exposure is a critical prerequisite for the emergence of activity in LHPenk neurons that encodes HFD salience.
We further explored the correlation between PSS-primed LHPenk neuronal activity and multiple eating bouts for HFD. After extracting in vivo Ca2+ transients from LHPenk neurons, we quantified the proportion of Ca2+ transients that occurred at the onset of eating bouts. Consistent with our previous data, we found that 76.6% of Ca2+ transients from PSS-primed LHPenk neurons were generated during HFD consumption (Supplementary Fig. 5d–f). In mice never exposed to PSS, only 38.81% of Ca2+ transients responded to the eating bouts (Supplementary Fig. 5a–c). These data support the hypothesis that PSS exposure potentiates the responsiveness of LHPenk neurons to HFD eating, which may lead to emotional overeating.
Modulation of LHPenk neuronal activity affects PSS-induced maladaptive behaviors
In light of our previous findings that increased activity of LHPenk neurons may encode PSS-induced behavioral changes (Fig. 3, Supplementary Fig. 4), we reasoned that selective activation of the LHPenk neurons may recapitulate the behavioral and emotional features of PSS mice, including HFD overconsumption and negative emotional valence. To test this idea, we bilaterally expressed Cre-dependent Gq-coupled designer receptors exclusively activated by designer drugs (DREADDs; hM3D) in the LH of Penk-Cre mice (Fig. 4a). In the presence of clozapine-N-oxide (CNO), an inert ligand specific to the DREADDs37, the mice expressing hM3D in LHPenk neurons showed significantly enhanced consumption of HFD but not NC, whereas the saline-treated mice exhibited no substantial differences in food intake levels (Fig. 4b, c and Supplementary Fig. 6a, b).
Fig. 4: Modulation of LHPenk neuronal activity affects PSS-induced maladaptive behaviors.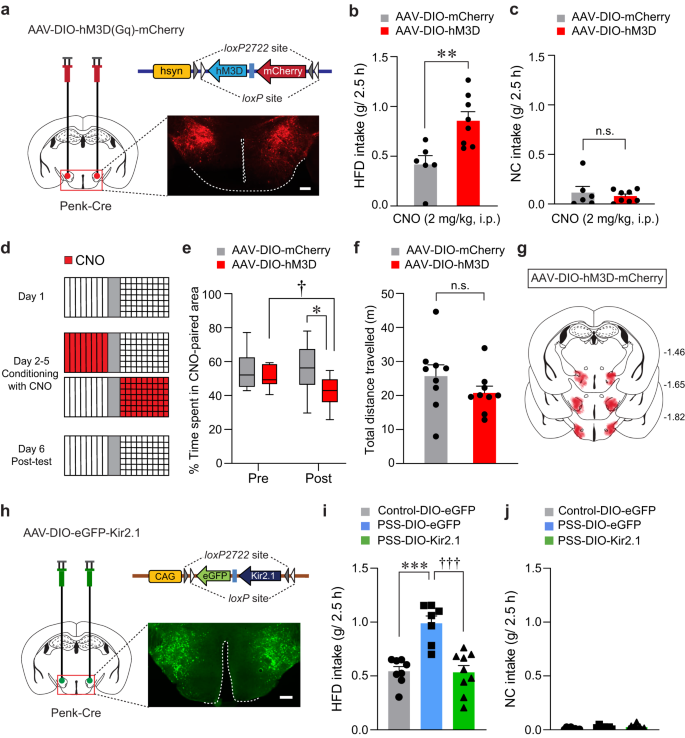
a Cre-dependent AAV expressing hM3D was injected in the LH of Penk-Cre mice, replicated independently with similar results in 4 mice. Scale bar, 250 μm. b, c CNO-induced activation of LHPenk neurons potentiates consumption of HFD, but not NC (n = 6, 8 mice for each group). In (b), two-tailed unpaired t test, t12 = −3.374, **p = 0.00552; In (c), two-tailed unpaired t test, t12 = 0.582, p = 0.571. d Experimental procedure to test CPA: one side of a two-sided chamber is paired with CNO injections (2 mg/kg, i.p) during day 2–5. e Time spent (%) in CNO-paired side of mice expressing DIO-mCherry or DIO-hM3D during pre- and post-test (n = 9 mice per group). Box–whisker plots display median (center) and 2.5 to 97.5 percentiles of the distribution (bounds) with whiskers extending from min to max values. Two-way RM ANOVA (F(1,16) = 5.011, p = 0.04) was followed by Bonferroni post hoc test for multiple comparisons; *p = 0.015 for mice with mCherry vs. hM3D during post-test; †p = 0.016 for hM3D mice during pre- vs. post-test. f Total distance traveled during the post-test of CPA experiment in (e) (n = 9 mice per group). Two-tailed unpaired t test, t11 = 1.660, p = 0.125. g Summary diagram for the coverage of hM3D-mCherry viral infusion in the LH of Penk-Cre mice in (a–f). h Cre-dependent AAV expressing Kir2.1 was injected in the LH of Penk-Cre mice, replicated independently with similar results in 5 mice. Scale bar, 250 μm. i, j Kir2.1-mediated inhibition of LHPenk neurons of PSS mice reduces the consumption of HFD, but not NC (n = 8, 7, and 9 mice per group). In (i), one-way ANOVA (F(2,21) = 17.730, p p †††p
Next, we asked whether chemogenetic activation of LHPenk neurons can transmit a negative valence signal. Using a CPA protocol (see the Methods; Fig. 4d), we found that mice expressing hM3D in LHPenk neurons exhibited a strong aversion to the CNO-paired chamber with regular locomotion (Fig. 4e–g), indicating the aversive nature of LHPenk neurons. Analogous results were obtained using a real-time place test (RTPT), with Penk-Cre mice expressing Channelrhodopsin2 (ChR2) in the LH followed by the implantation of an optic cannula in the same site (Supplementary Fig. 6c). Unlike control Penk-Cre mice expressing eYFP alone, the ChR2-expressing Penk-Cre mice avoided the side of the RTPT apparatus paired with photostimulation (10-ms pulses of 473-nm light at 20 Hz) with no accompanying change in locomotion (Supplementary Fig. 6d–f). Moreover, ChR2-mediated activation of LHPenk neurons at the same frequency (20 Hz) significantly increased the HFD consumption without affecting NC intake (Supplementary Fig. 6g, h). Together, these data suggest that activation of LHPenk neurons increases the tendency of mice to overconsume palatable foods, which is possibly triggered by an aversive or negative emotional state.
These results led us to hypothesize that silencing LHPenk neurons could reverse the emotional overeating and negative emotional valence associated with PSS exposure. To suppress LHPenk neuronal activity in vivo, we injected the LH of Penk-Cre mice with AAV expressing the Cre-dependent Kir2.1 potassium channel38 (AAV-DIO-eGFP-Kir2.1) (Fig. 4h). Twenty-four hours after PSS exposure, we confirmed that chronic silencing of LHPenk neurons significantly attenuated HFD overconsumption compared to mice expressing eGFP alone (Fig. 4i). Importantly, this Kir2.1-mediated LHPenk neuronal inhibition did not affect NC intake (Fig. 4j) or cause any accompanying changes in locomotion or olfactory perception (Supplementary Fig. 6i–k). These data suggest that inhibition of LHPenk neurons can alleviate the palatable food overconsumption triggered by PSS.
We further investigated whether LHPenk neuronal inhibition is sufficient to prevent the negative emotional states that follow PSS exposure. We subjected mice expressing Kir2.1 in the LHPenk neurons to a CPA paradigm paired with PSS and monitored their behaviors (Supplementary Fig. 6l). We found that while eYFP-expressing controls spent less time in the PSS-paired chamber, mice expressing Kir2.1 did not show avoidance behaviors (Supplementary Fig. 6m–o). Together with these results, we speculated that Kir2.1-mediated inhibition of LHPenk neurons attenuates the palatable food overconsumption associated with a negative emotional state that we observed in PSS mice.
Roles of enkephalinergic systems in the LH circuitry on PSS-induced maladaptive changes
Enkephalins are produced from a propeptide precursor, Penk mRNA, which encodes distinct form of endogenous opioid peptides39. We therefore investigated the functional roles of endogenous enkephalin in the LH and the related circuit elements in mediating the effects of PSS on emotional eating. Given previous studies indicating emerging roles of enkephalin signaling in adaptation to stressful experiences40, we asked whether the Penk in the LH is necessary for HFD overconsumption after PSS exposure. To test this, we bilaterally injected AAV carrying a short hairpin RNA (shRNA) against Penk in a Cre-dependent manner (AAV-DIO-EmGFP-Penk shRNA) into the LH of Penk-Cre mice and confirmed a significant reduction of Penk mRNA expression without accompanying changes in the Lepr, Hcrt and Pmch (Fig. 5a, b). At 24 h post-PSS exposure, we found that shRNA-mediated knockdown of Penk significantly reduced HFD consumption, but not NC intake, whereas PSS mice injected with control virus (AAV-DIO-eGFP) showed HFD overconsumption (Fig. 5c–e). These results indicate that downregulation of Penk in the LH has a critical role in the protection against PSS-induced HFD overconsumption.
Fig. 5: Endogenous enkephalin in the LH is necessary for PSS-induced maladaptive responses.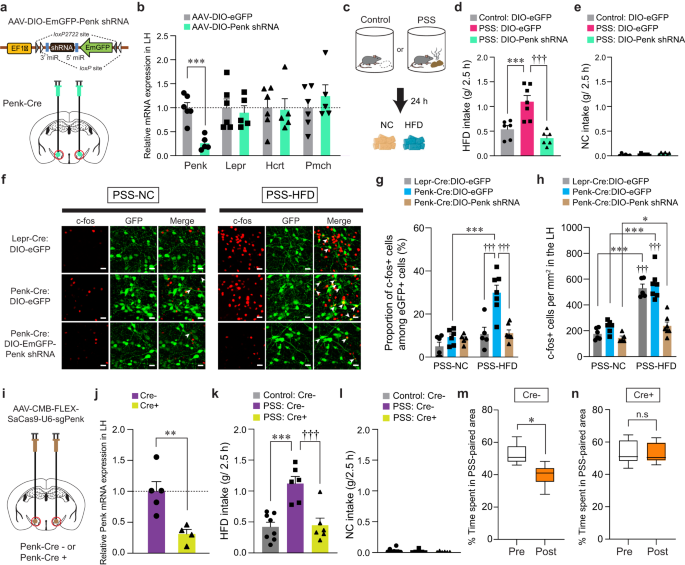
a, b Schematic for the injection of AAV-DIO-Penk shRNA into the LH (a). Red circles denote the dissected regions used in RT-qPCR (b; n = 6, 5 mice per group). In (b), two-tailed unpaired t test, t9 = 5.589, ***p = 0.000339. c–e 2.5 h food consumption test (n = 6, 7, 6 mice per group). In (d), one-way ANOVA (F(2,16) = 16.713, p p †††p f Representative images showing c-fos immunoreactivity and eGFP in the LH of Lepr-Cre and Penk-Cre mice. Arrowheads indicate the colocalization of c-fos with eGFP. Scale bars, 20 μm. g, h Quantification of the proportion of c-fos+ cells among eGFP-labeled LH neurons (g) or c-fos+ cells in the LH (h) (n = 6, 6, 5 mice for each PSS-NC group; n = 5, 7, 7 mice for each PSS-HFD group). In (g), two-way ANOVA (F(2,30) = 8.543, p = 0.001) was followed by Fisher LSD post hoc test for multiple comparisons; ***p †††p h), two-way ANOVA (F(2,30) = 11.261, p p = 0.034, ***p †††p i, j Schematic for CRISPR virus injections into the LH (i). Red circles denote the dissected LH used in RT-qPCR (j; n = 5, 4 mice per group). In (j), two-tailed unpaired t test, t7 = 3.631, **p = 0.00839. k, l 2.5 h food consumption test (n = 8, 6, 6 mice per group). In (k), one-way ANOVA (F(2,17) = 15.477, p p †††p m, n Time spent (%) in PSS-paired side of mice receiving CRISPR virus in the LH (n = 6 mice). Box–whisker plots display median (center) and 2.5 to 97.5 percentiles of the distribution (bounds) with whiskers extending from min to max values. In (m), two-tailed paired t test, t5 = 3.892, *p = 0.0115; In (n), two-tailed paired t test, t5 = 0.186, p = 0.86. n.s not significant. Data are expressed as mean ± SEM.
Because our previous data showed PSS mice displaying enhanced expression of c-fos in the LH in response to HFD eating (Supplementary Fig. 1a, b), we hypothesized that enkephalin-mediated signaling is necessary to potentiate the responsiveness of PSS-primed LHPenk neurons during emotional overeating. We first asked whether the HFD-induced expression of c-fos observed in PSS mice mainly occurs in the LHPenk neurons compared to Lepr-expressing LH (LHLepr) neurons representing mostly distinct subpopulation from the LHPenk neurons (Fig. 2h). We injected AAV-DIO-eGFP into the LH of Penk-Cre or Lepr-Cre mice to visualize LHPenk and LHLepr neuronal cell bodies, respectively, and then measured the proportion of c-fos-positive LH cells 24 h after PSS exposure. In response to HFD, we found enhanced c-fos induction in the LHPenk neuronal population by up to 29.91%, but to a lesser extent, only 10.72% of c-fos expression were overlapped with eGFP-expressing LHLepr cells (Fig. 5f, g). Under the same condition, the total number of c-fos-positive cells in the overall LH was increased consistently in both groups, regardless of cell-type specific eGFP-labeling (Fig. 5h). These data suggest that LHPenk neurons constitute a more relevant population than LHLepr neurons in controlling PSS-induced HFD overconsumption. Importantly, shRNA-mediated knockdown of Penk significantly decreased HFD-induced c-fos expression in the PSS-primed LH, particularly within the LHPenk neurons (Fig. 5f–h), confirming that the enkephalin peptide plays a critical role in increasing LHPenk neuronal reactivity to HFD, which is a hallmark of PSS mice exhibiting emotional overconsumption.
To further confirm the necessity of endogenous enkephalin in the LH, we used CRISPR-SaCas9 viral-based system by bilaterally injecting AAV-CMV-FLEX-SaCas9-U6-sgPenk18 into the LH of Penk-Cre mice (Fig. 5i). Similar to the effects of shRNA-mediated knockdown of Penk, CRISPR infection reduced Penk expression by 70% (Fig. 5j) and behaviorally suppressed PSS-induced HFD overconsumption without changing NC intake (Fig. 5k, l). Furthermore, in the CPA paradigm, CRISPR-mediated reduction of Penk in the LH resulted in decreasing behavioral avoidance for PSS-paired chamber (Fig. 5m, n), suggesting that LH endogenous enkephalin is necessary for mediating PSS-induced maladaptive behavioral responses including HFD overconsumption and associated negative emotional state.
Because enkephalins are potent endogenous agonists binding to MORs, we next asked whether MORs in the LHPenk neuronal efferent projections are the receptor targets that relay the signals from the enkephalin-containing LH neurons. To delineate the efferent projections of LHPenk neurons, we injected AAV-DIO-eGFP into the LH of Penk-Cre mice and found that LHPenk neurons send projections to the ventral pallidum (VP), VTA, substantia nigra (SN), periaqueductal gray (PAG), and lateral parabrachial nucleus (LPBN) (Fig. 6a–c). Among these labeled regions, the PAG, one of the brain regions thought to be responsible for directing the consumption of innately palatable substances41,42, receives a dense LHPenk axonal projection particularly into the lateral part (LPAG). Since MORs are highly expressed in the same area43, we reasoned that MORs in the LPAG are necessary for triggering HFD overconsumption after artificial activation of LHPenk neurons. We tested this idea by chemogenetically activating LHPenk neurons and simultaneously inhibiting MORs through intracerebral microinjections of the MOR-selective antagonist CTAP (0.1 or 1 μg per side) into the LPAG (Fig. 6d, e). Consistent with our previous findings (Fig. 4), CNO-mediated activation of LHPenk neurons significantly increased HFD consumption (g/2.5 h). However, a local infusion of CTAP into the LPAG, particularly at a high dose (1 μg per side), strongly blocked the HFD consumption even in the presence of CNO, while mice in all groups showed regular NC intake (Fig. 6f, g). Notably, a microinfusion of CTAP (1 μg per side) into the LPAG did not affect general locomotion (Fig. 6h), suggesting that the pharmacological inhibition of MORs in the LPAG specifically suppresses HFD eating behaviors driven by chemogenetic activation of LHPenk neurons.
Fig. 6: Pharmacological inhibition of MORs in the LPAG reduces HFD consumption.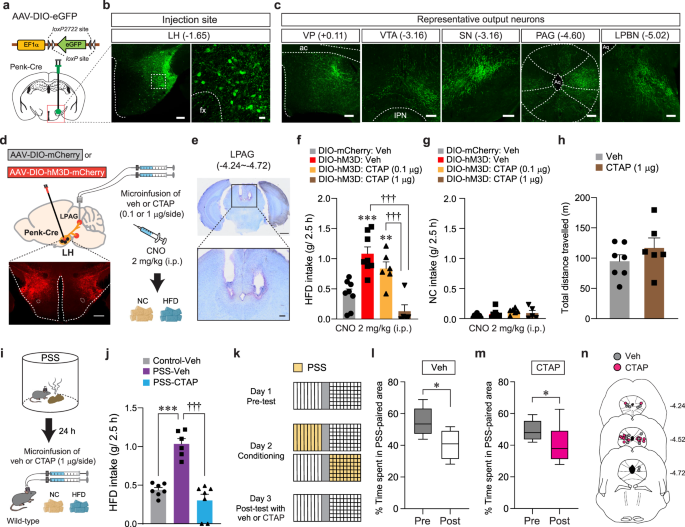
a Schematics for the injection of AAV-DIO-eGFP into the LH of Penk-Cre mice. b Images showing eGFP expression in the LHPenk neuronal cells (left), and the high magnification image of a region in the white dashed box (right), replicated independently with similar results in 3 mice. Scale bar, 200 μm, 25 μm, respectively. fx, fornix. c eGFP-positive axons from LHPenk neuron were found in the VP, VTA, SN, PAG, and LPBN, replicated independently with similar results in 3 mice. Scale bars, 200 μm. ac, anterior commissure; IPN, interpeduncular nucleus; Aq, aqueduct. d Schematic for microinjections of CTAP into the LPAG of mice expressing mCherry or hM3D in the LHPenk neurons. Confocal image showing hM3D-expressing LHPenk neurons, replicated independently with similar results in 5 mice. Scale bar, 500 μm. e Images show the location of the cannula tips in the LPAG, replicated independently with similar results in 5 mice. Scale bars, 1 mm (top) and 200 μm (bottom). f, g 2.5 h food consumption (n = 8, 8, 6, 5 mice per group). In (f), one-way ANOVA (F(3,23) = 15.946, p p = 0.008, ***p †††p h Locomotor activity (n = 7, 6 mice per group). i Schematic for microinjection of veh or CTAP into the LPAG of PSS mice before HFD exposure. j 2.5 h HFD consumption test (n = 7, 6, 7 mice per group). One-way ANOVA (F(2,17) = 35.847, p p †††p k–m Time spent (%) in the PSS-paired chambers (n = 7, 8 mice per group). Box–whisker plots display median and 2.5 to 97.5 percentiles of the distribution with whiskers extending from min to max values. In (l), two-tailed paired t test, t6 = 2.776, *p = 0.0322. In (m), two-tailed paired t test, t7 = 2.978, *p = 0.0206. n Locations of the cannula tips in the mice included in (l, m). Data are expressed as mean ± SEM.
Because activation of LHPenk neurons is a critical component to mediate PSS-induced HFD overconsumption (Fig. 4), we also asked whether the pharmacological inhibition of MORs in the LPAG can normalize maladaptive behavioral responses developed after PSS exposure. Indeed, at 24 h post-PSS exposure, intracranial microinfusion of CTAP (1 μg per side) into the LPAG prevented PSS mice from showing augmented eating upon HFD exposure (Fig. 6i, j). In contrast to this, the same pharmacological inhibition of MORs in the LPAG did not affect aversive behavioral responses for PSS-paired compartment in the CPA procedure (Fig. 6k–n). These data suggest that the activation of MOR signaling in the LPAG is preferentially required for eliciting PSS-induced HFD overconsumption, but not negative emotional state. Together, these results support that endogenous enkephalinergic system (e.g., enkephalin peptides and their receptors) in the LH circuitry is important for regulating the tendency to develop palatable food overconsumption, substantiating the unique role of LHPenk neurons in mediating post-PSS effects.
CORT mimics the delayed onset of emotional overeating after PSS
The HPA system plays a primary role in coordinating endocrine and behavioral responses to stress. Activation of the HPA axis results in the release of corticotropin-releasing hormone (CRH) from the PVN. This, in turn, increases glucocorticoid hormone levels (primarily corticosterone in rodents and cortisol in humans), which can cause lasting changes in neuronal function and behaviors23,44,45. Notably, we found that while serum CORT levels are significantly elevated 30 min after PSS exposure, they return to baseline 24 h later (Fig. 7a, b).
Fig. 7: CORT increases HFD intake and LHPenk neuronal reactivity to HFD consumption.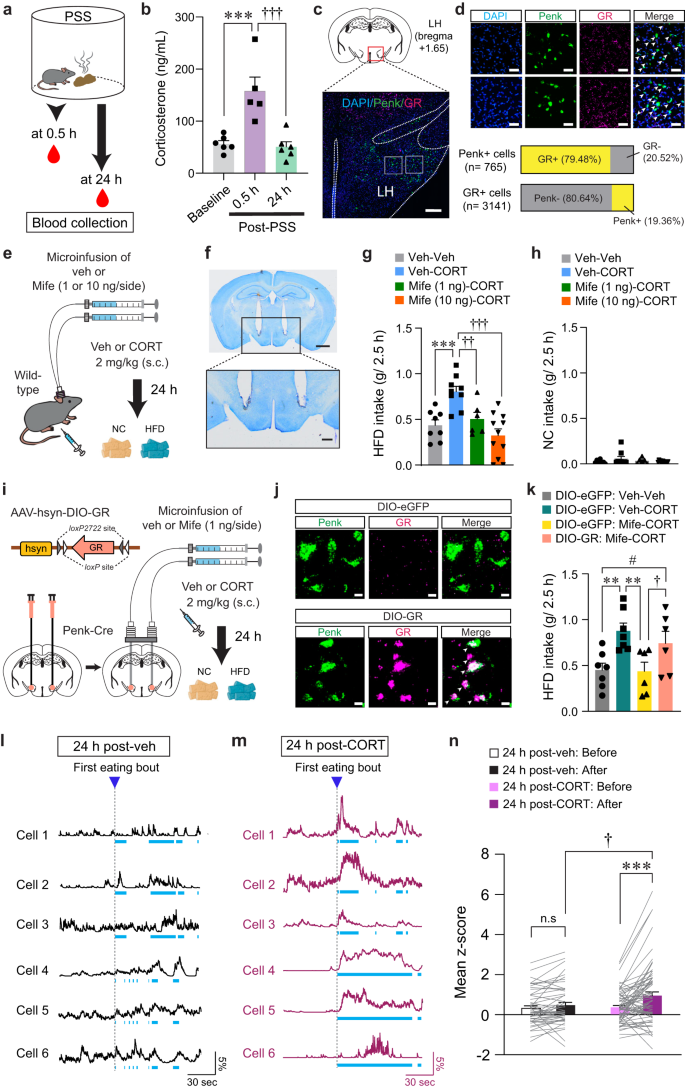
a, b Serum CORT levels after PSS (b; n = 6, 5, and 6 mice). In (b), One-way ANOVA (F(2,14) = 14.634, p p †††p c Representative image of FISH for Penk and GR in the LH, replicated independently with similar results in 3 mice. Scale bars, 250 μm. d Enlarged views of regions in the white boxes showing in (c), replicated independently with similar results in 3 mice. Scale bars, 50 μm. Arrowheads represent colocalization (top). Bar charts indicate % of Penk+ or GR + LH neurons colocalizing with GR or Penk, respectively (bottom). e Schematic for the microinjection of vehicle or mifepristone into the LH followed by systemic injections. f Images show the location of the cannula tips in the LH, replicated independently with similar results in 6 mice. Scale bars, 1 mm (top) and 250 μm (bottom). g, h 2.5 h food consumption (n = 8, 9, 6, and 11 mice). In (g), one-way ANOVA (F(3,30) = 10.336, p p ††p = 0.007, †††p i Schematic for the bilateral injection of AAV-DIO-GR into the LH, followed by implantation of cannula. j Representative images of FISH for Penk and GR in the LH of Penk-Cre mice expressing DIO-eGFP (top) and DIO-GR (bottom). Arrowheads represent colocalization, replicated independently with similar results in 2 mice. Scale bars, 10 μm. k 2.5 h HFD consumption test (n = 7, 7, 6, and 6 mice). One-way ANOVA (F(3,22) = 5.149, p = 0.008) was followed by Fisher LSD post hoc test for multiple comparisons; **p = 0.004, **p = 0.004 compared with DIO-eGFP mice received veh (μ-injection) and CORT (s.c.); †p = 0.045, #p = 0.047 compared with DIO-GR mice received Mife (μ-injection) and CORT (s.c). l, m Example in vivo Ca2+ traces in response to HFD eating. Light blue dashed areas indicate eating bouts. n Mean z-score of the LHPenk neuronal activity in veh- (n = 51 cells from 5 mice) or CORT-treated mice (n = 68 cells from 5 mice). Two-way RM ANOVA (F(1,116) = 6.141, p = 0.015) was followed by Bonferroni post hoc test for multiple comparisons; ***p †p = 0.021 compared with CORT-treated mice after 1st HFD eating bout; p = 0.27 for veh-treated mice before vs. after 1st HFD eating bout. n.s not significant. Data are presented as mean ± SEM.
To understand whether CORT promotes emotional overeating via directly acting on LHPenk neurons, we first asked whether LHPenk neurons express the CORT receptors such as GR or mineralocorticoid receptor (MR)46. We found that most LHPenk neurons expressed GR (79.48%) rather than MR (Fig. 7c, d, and Supplementary Fig. 7a), suggesting that a substantial fraction of LHPenk neurons can convey CORT signals through GR.
We next sought to determine whether mice treated with CORT exhibit HFD overconsumption previously seen in PSS mice. Immediately after CORT administration (2 mg/kg, s.c.), we did not observe any changes in HFD or NC intake. Twenty-four hours after CORT injection, however, mice showed HFD overconsumption without any change in NC intake (Supplementary Fig. 7b, c), recapitulating the PSS-driven delayed onset of overeating. Since most LHPenk neurons express GR (Fig. 7c), we hypothesized that GR activation in the LH is required for the CORT-induced HFD overconsumption. To test this hypothesis, we performed an intracranial microinfusion of the GR antagonist, mifepristone (1 or 10 ng per side) into the LH followed by systemic injections of vehicle or CORT (Fig. 7e, f, and Supplementary Fig. 7d). On the next day, we observed that mifepristone microinfusion prevented the CORT pretreatment from increasing HFD intake in a dose-dependent manner with no accompanying changes in NC intake (Fig. 7g, h). We did not observe any differences in locomotion of the mifepristone-microinjected mice (Supplementary Fig. 7e), suggesting the normalization of HFD overconsumption we observed with mifepristone microinfusion cannot be ascribed to altered movements. Together, these data indicate that activation of GR signaling in the LH is necessary for mediating the delayed onset of HFD overconsumption after CORT treatments.
To further confirm the active role of the GR specifically expressed in the LHPenk neurons, we asked whether LHPenk neuron-specific overexpression of GR can reverse the effect of mifepristone in the LH of CORT-treated mice. We stereotaxically injected AAV expressing GR in a Cre-dependent manner (AAV-hsyn-DIO-GR) into the LH of Penk-Cre mice, followed by implantation of drug-infusion cannulae above the virus injected site (Fig. 7i). We confirmed that GR mRNA levels were robustly increased both in FISH experiments and quantitative real-time PCR (qRT-PCR) assay (Fig. 7j and Supplementary Fig. 7f). We also found that the specific overexpression of GR in the LHPenk neurons blocked the mifepristone-induced normalization of HFD overconsumption in CORT-treated mice (Fig. 7k), suggesting that inhibition of GR signaling in the LHPenk neurons is a critical component for mifepristone to suppress the effects of CORT on triggering palatable food overconsumption.
To determine how CORT regulates the basal activity of LHPenk neurons, we next virally expressed Cre-dependent GCaMP6f in the LH of Penk-Cre mice and monitored in vivo Ca2+ dynamics following systemic CORT injection (2 mg/kg, s.c.). Five minutes after CORT administration, we observed a decrease in the average number of Ca2+ transients per minute in LHPenk neurons, which was recovered shortly to the level of one in vehicle-treated animals (Supplementary Fig. 7g, h). Twenty-four hours after CORT treatment, we no longer observed any difference in the baseline activity of the LHPenk neurons (Supplementary Fig. 7i–l). These data demonstrate that acute CORT treatment induces a transient reduction in baseline LHPenk neuronal activity in vivo, which was followed by a rapid recovery.
We next explored how CORT pretreatment affects the in vivo Ca2+ dynamics of LHPenk neurons during HFD consumption. Twenty-four hours after drug administration, we found that mice treated with CORT showed higher LHPenk neuronal reactivity at the first onset of HFD eating bout compared to vehicle-treated mice (Fig. 7l–n). Furthermore, we investigated whether CORT pretreatment increases the HFD-induced expression of c-fos in the LHPenk neurons. With CORT pretreatment, HFD increased the proportion of c-fos–positive LHPenk neurons up to 31.6%, but vehicle-treated mice exhibited only 7.4% and 11.16% of LHPenk neurons c-fos–positive upon NC or HFD exposure, respectively (Supplementary Fig. 8a–c). Moreover, a local infusion of mifepristone into the LH prior to CORT administration led to significant reduction in c-fos–positive LHPenk neurons (5.48%) after HFD exposure, confirming that the HFD-induced c-fos expression was mediated by GR activation in the LH after CORT treatment (Supplementary Fig. 8d–f). Together, these results support that CORT pretreatment can increase the responsiveness of the LHPenk neurons to HFD eating, which is a hallmark previously seen in PSS mice (Fig. 3).
Inhibition of GR in the LH reverses PSS-induced maladaptive changes
Our results led us to hypothesize that PSS-induced CORT elevation may alter LHPenk neuronal responsiveness to HFD via activation of GR signaling, leading to emotional overeating. We therefore asked whether pharmacological inhibition of GR signaling in the LH normalizes the maladaptive eating and negative emotional behaviors that follow PSS exposure. We performed an intracranial microinfusion of either vehicle or mifepristone (1 or 10 ng per side) into the LH before PSS exposure (Fig. 8a, b, and e). Twenty-four hours later, we found that PSS mice that received mifepristone showed a dose-dependent decrease in HFD consumption (Fig. 8c). Regardless of drug treatment, we did not observe changes in NC consumption in any of the groups (Fig. 8d). These results suggest GR activation in the LH is necessary for PSS-induced HFD overconsumption.
Fig. 8: Inhibition of GR signaling in the LH prevents PSS-induced maladaptive behaviors.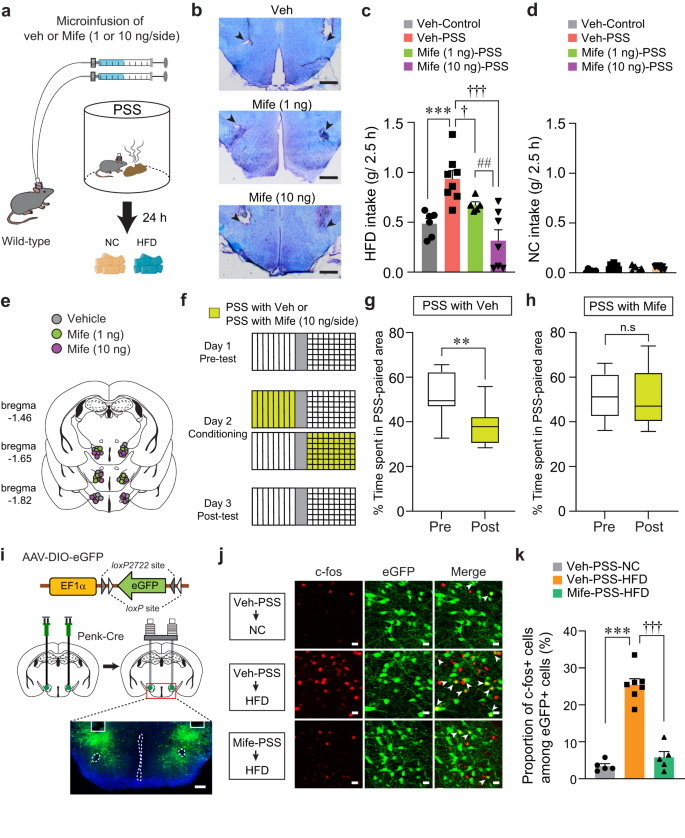
a Schematics for drug microinfusions before PSS exposure. b Images show locations of cannula tips in the LH. Arrowheads denote the end of tips, replicated independently with similar results in 4 mice. Scale bars, 500 μm. c, d 2.5 h food consumption 24 h after exposure to either control or PSS (n = 6, 8, 5, and 7 mice). In (c), one-way ANOVA (F(3,22) = 11.778, p p †p = 0.041, †††p ##p = 0.008 compared with Mife (10 ng/side)-injected PSS mice. e Locations of the injection cannula tips in the mice included in (c, d). f–h Experimental procedure to test CPA (f). Time spent (%) of veh- (g; n = 8 mice) and Mife-treated mice (h; n = 6 mice) in PSS-paired chambers during pre- and post-test. Box–whisker plots display median (center) and 2.5 to 97.5 percentiles of the distribution (bounds) with whiskers extending from min to max values. In (g), two-tailed paired t test, t7 = 4.380, **p = 0.00324. In (h), two-tailed paired t test, t5 = 0.476, p = 0.654. i Schematic for the bilateral injection of AAV-DIO-eGFP, followed by implantation of cannula into the LH of Penk-Cre mice, replicated independently with similar results in 5 mice. Scale bar, 200 μm. j Representative images showing c-fos immunoreactivity and eGFP in the LH in response to either NC or HFD 24 h after PSS exposure. White arrowheads indicate the colocalization of c-fos with Penk expression, replicated independently with similar results in 5 mice. Scale bars, 20 μm. k Quantification of the proportion of c-fos-positive cells among LHPenk neurons in (j) (n = 5, 7, and 5 mice per group). One-way ANOVA (F(2,14) = 71.860, p p †††p
To determine whether pharmacological inhibition of GR in the LH blocks the negative emotional state that follows PSS exposure, we performed a place preference conditioning test. We delivered mifepristone into the LH of mice before subjecting them to a PSS-paired side chamber on day 2 (Fig. 8f). On test day, we found that the local infusion of mifepristone alleviated the PSS-induced post-conditioning avoidance, whereas vehicle-treated mice spent less time in the chamber paired with PSS (Fig. 8g, h). Together, these data suggest that GR signaling inhibition in the LH can normalize the HFD overconsumption and aversive emotional state induced by PSS exposure.
Given our previous data showing that increased LHPenk neuronal activity is likely to encode PSS-induced HFD overconsumption (Figs. 3 and 4), we next asked whether inhibition of GR signaling blocks the sensitized LHPenk neuronal reactivity of PSS mice upon HFD exposure. After injecting AAV-DIO-eGFP into the LH of Penk-Cre mice, we implanted a cannula into the same site for the local infusion of mifepristone (Fig. 8i). We found that up to 25.42% of LHPenk neurons show HFD-induced c-fos expression in vehicle-treated PSS mice, but a local infusion of mifepristone reduced the induction of c-fos, resulting in only 5.78% of LHPenk neurons showing HFD-induced c-fos expression (Fig. 8j, k). These data support the hypothesis that PSS-driven activation of GR signaling in the LH is required for sensitizing LHPenk neuronal activity to HFD, leading to the emotional overeating toward palatable food following PSS exposure.