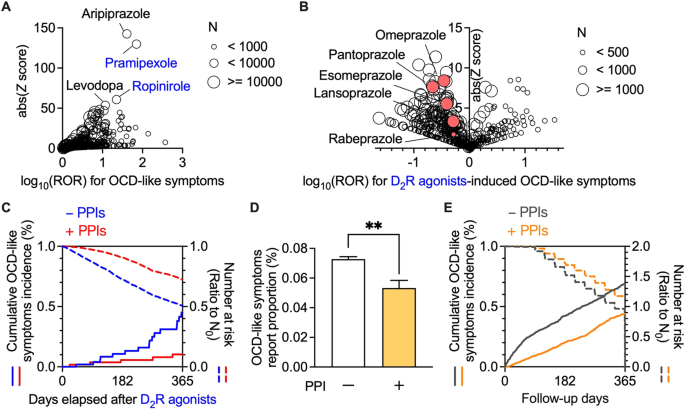
Concomitant use of PPIs suppressed the incidence of OCD-like symptoms induced by D2R agonists
We first analysed FAERS data to investigate the association between drug use and OCD-like symptoms (Supplementary Table 1). A disproportionality analysis revealed a significant association between the use of dopamine receptor stimulants and increased occurrence of OCD-like symptoms (Fig. 1A and Supplementary Table 2). Among the dopamine receptor stimulants, pramipexole, and ropinirole, which are D2R agonists, showed high reporting odds ratios (ROR) and Z scores. Moreover, aripiprazole, widely used as an augmentation therapy for patients with treatment-resistant OCD, was strongly associated with the emergence of OCD-like symptoms [23]. We observed a relationship between the use of D2R agonists and OCD-like symptoms through a comprehensive analysis of FAERS data.
Next, we evaluated the confounding effects of all drug combinations among D2R agonists users (Fig. 1B and Supplementary Table 3). Among the concomitant drugs which decreased the incidence of OCD-like symptoms induced by D2R agonists, a class effect was observed in the following three drug groups: vitamin D (cholecalciferol and ergocalciferol), loop diuretics (furosemide, torsemide, and bumetanide), and proton pump inhibitors (PPIs; pantoprazole, omeprazole, esomeprazole, lansoprazole, and rabeprazole). Vitamin D was excluded, given its therapeutic effect on restless legs syndrome, which is frequently treated with D2R agonists [24], as it may reduce the dosage of D2R agonists. Furthermore, loop diuretics were omitted since renal dysfunction can affect the pharmacokinetics of pramipexole, which is mainly excreted in the urine [25]. Accordingly, we focused on PPIs as a potential concomitant drug inhibiting OCD-like symptoms.
We subsequently analysed the effects of the use of PPIs and the onset of OCD-like symptoms induced by D2R agonists to overcome the reporting bias and lack of a denominator for incidence because of self-reporting. This analysis used MarketScan data, which contains information on the number of D2R agonists users and their prescription dates (Supplementary Fig. 1A, B and Supplementary Tables 4–6).
Patients who received PPIs were defined as those prescribed PPIs for the first time after using D2R agonists. We performed 1:1 propensity score matching (Supplementary Tables 7–11) to eliminate known confounding factors [2, 26,27,28].
We analysed the daily doses, cumulative doses, and administration periods of D2R agonists, levodopa, and PPIs to validate the matched cohorts (Table 1). The doses of D2R agonists and PPIs were converted to the levodopa equivalent dose (LED) [29] and omeprazole equivalent dose (OED) [30], respectively. The daily doses of D2R agonists and PPIs were within the standard dose range in the USA (D2R agonists: LED 12.5–450, PPIs: OED 5–40).
Table 1 Daily and cumulative doses and administration periods of D2R agonists, levodopa, and proton pump inhibitors (PPIs) in the propensity score-matched cohorts when used in combination with proton pump inhibitors of MarketScan data using l-dopa equivalent dose (LED) and omeprazole equivalent dose (OED).
Bias toward the levodopa doses may have affected the analysis because levodopa use is associated with OCD-like symptoms [31]. In both cohorts, the levodopa dose was reasonable (standard dose: LED 42–500), with no between-group difference in dose.
We performed a chronological sequence analysis to assess the inhibitory effects of PPIs on OCD-like symptoms induced by D2R agonists. Consistent with the FAERS analysis, Kaplan-Meier analysis, and Cox proportional hazards modelling indicated that the concomitant use of PPI(s) significantly decreased the incidence of OCD-like symptoms induced by D2R agonists, with a hazard ratio of 0.432 (Fig. 1C, 95% CI: 0.238–0.783, P = 0.004 in the log-rank test).
Concomitant use of H2 blocker(s) did not significantly affect the incidence of OCD-like symptoms induced by D2R agonists (Supplementary Fig. 1C, a hazard ratio of 1.10, 95% CI: 0.359–3.39, P = 0.090 in the log-rank test; Supplementary Tables 12–17), suggesting that the inhibitory effects of PPIs on the incidence of OCD-like symptoms did not result from the inhibition of gastric acid secretion and history of gastrointestinal diseases.
Furthermore, we investigated whether the administration of PPIs was effective for OCD-like symptoms in patients without D2R agonists. Analysis of FAERS data revealed that the use of PPIs significantly reduced the incidence of OCD-like symptoms in non-D2R agonists users (Fig. 1D). Additionally, in matched cohorts among non-D2R agonists users of MarketScan data, PPIs also significantly lowered the incidence of OCD-like symptoms with a hazard ratio of 0.533 (Fig. 1E, 95% CI: 0.483–0.589, P 18 and 19).
These findings showed that the concomitant use of PPIs alleviated OCD-like symptoms.
PPI ameliorated behavioural repetition induced by repeated D2R stimulation
Repeated injections of a D2R agonist (QNP) elicit OCD-like behavioural and neurological abnormalities in mice [12, 13, 17]. We assessed the effects of PPIs on QNP-induced OCD-like behaviours to obtain preclinical proof-of-concept. We previously showed that repeated injection of QNP induced repetitive behaviour [12, 13, 17]. Consistent with our previous observations, repeated intraperitoneal injection of QNP (1 mg/kg/day, eight times) significantly induced excessive repetition of spontaneous chewing behaviour (Fig. 2A, B). When mice were treated with lansoprazole (100 mg/kg) for four days before QNP administration (Fig. 2C), the time spent chewing was significantly decreased (Fig. 2D).
Fig. 2: Vonoprazan administration improved repetitive behaviour in quinpirole-treated mice.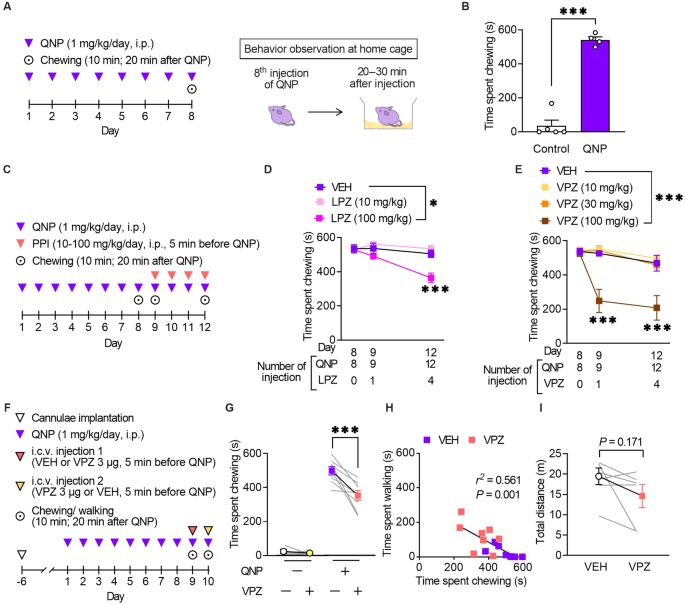
A Mice received eight daily injections of quinpirole (QNP; D2R agonist; 1 mg/kg), and spontaneous behaviour was observed 20–30 min after the eighth injection of QNP. B The time spent chewing in the vehicle (control) or QNP-treated mice within 10 min. C Mice received intraperitoneal injections of a proton pump inhibitor (PPI) 5 min before the 9–12th QNP injection. Chewing was measured for 10 min starting from 20 min after QNP administration. D Time spent chewing in mice received lansoprazole (LPZ) for four days. E Time spent chewing in mice received vonoprazan (VPZ) for 4 days. F QNP injections were started seven days after cannula implantation into the cerebral ventricle. VEH or VPZ (3 μg) was injected through the cannula 5 min before the 9th or 10th QNP injection in a crossover design for the initial measurement of chewing and walking behaviour. G The effect of intracerebroventricular administration of VPZ (3 μg) on QNP-induced chewing behaviour. H Correlation between chewing and walking in mice treated with VEH or VPZ. r2 = 0.561, P = 0.001. I Effect of intracerebroventricular administration of VPZ (3 μg) on locomotor activity. *P P
We validated the effects of vonoprazan, a potassium-competitive acid blocker with more rapid inhibition of P-type proton pumps (the main PPI targets) than conventional PPIs to identify the class effect of PPI [32]. Similarly, vonoprazan (100 mg/kg) inhibited the QNP-induced increase in repeated chewing behaviour (Fig. 2E). In contrast to lansoprazole, a single administration of vonoprazan exerted an inhibitory effect.
Mice received vonoprazan injections in the intracerebroventricular space to exclusively assess the effects of PPIs in the brain. Consistent with systemic vonoprazan administration, a single administration of vonoprazan (3 μg) ameliorated QNP-induced repeated chewing behaviour (Fig. 2F, G). Time spent chewing was negatively correlated with time spent walking (Fig. 2H). Intracerebroventricular injection of vonoprazan (3 μg) did not affect locomotor activity, demonstrating that the decreased chewing behaviour did not result from the sedative effect of vonoprazan (Fig. 2I).
PPI ameliorated habitual action control induced by repeated D2R stimulation
Maladaptive habit-based decision-making and excessive/inappropriate repetition of actions are critical clinical characteristics of patients with OCD and OCD-like symptoms [1]. Recent animal studies have assessed OCD-like behavioural abnormalities through the facilitated formation of habitual behaviour [33, 34].
The formation of habitual strategies in QNP-treated mice was assessed using the devaluation test following a self-paced operant conditioning task (Fig. 3A). We also assessed the effects of long-term administration of an SSRI, the first-choice treatment for OCD to evaluate the clinically relevant treatment response [2]. The 6-day training on the random ratio (RR) reinforcement schedule gradually increased the lever press rates in all treatment groups, which suggested successful learning. However, there were between-group differences in the late training phase (Fig. 3B).
Fig. 3: Vonoprazan administration improved habitual action in quinpirole-treated mice.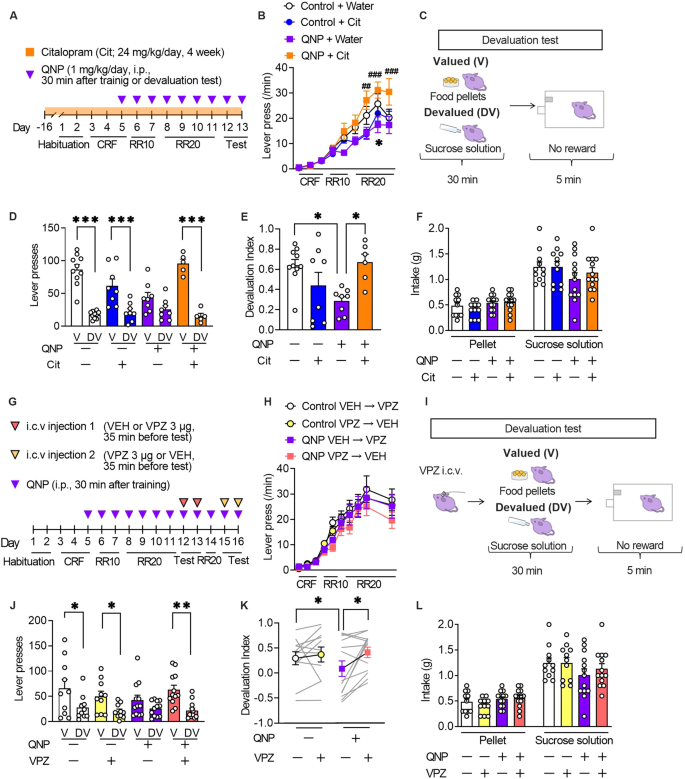
A The mice underwent 11-day training on the random ratio (RR) schedule, followed by 2-day outcome devaluation testing. Mice were treated daily with QNP (1 mg/kg) for eight days; moreover, they orally received water or citalopram (24 mg/kg/day; Cit) for four weeks before the first outcome devaluation test. B Response speed of lever pressing during acquisition under operant training schedules. *P ##P ###P C In the devaluation test, mice were fed food pellets on the valued (V) day or sucrose solution on the devalued (DV) day for 30 min. After pre-feeding, a 5-min extinction test was performed. D, E The numbers of lever presses in the devaluation tests (D) and devaluation index (E). F Consumption of food pellets and sucrose solution during the 30-min free-feed period. G Mice underwent 11-day training on the random ratio (RR) schedule and were treated daily with QNP (1 mg/kg) for 8 days before the first outcome devaluation testing. Vehicle (VEH) or vonoprazan (VPZ; 3 µg) was injected for outcome devaluation testing on day 12 or 13. On day 14, the mice were retrained to press the lever. On day 15 or 16, the second set of outcome devaluation testing was performed in a crossover design. H The response rate of lever pressing during acquisition under operant training schedules. I On the days of devaluation tests, mice received an intracerebroventricular injection of VEH or VPZ (3 μg) 5 min before the free-feeding session. J, K The numbers of lever presses in the devaluation tests (J) and devaluation index (K). L Consumption of food pellets and sucrose solution in the mice during the 30-min free-feed period. *P P P
The 6-day training on an RR schedule is widely used to develop goal-directed strategies for lever press [18]. In a goal-directed strategy, reduction in the reinforcer value by satiety (devaluation) decelerates lever presses in the subsequent 5-min unrewarded lever press session, with a lack of a decelerating effect suggesting the formation of a habitual strategy (Fig. 3C). Vehicle-treated control mice showed a significant reduction in the number of lever presses in the devalued condition, which suggested the successful formation of a goal-directed strategy. QNP-treated mice showed relatively similar lever pressing rates between the valued and devalued states compared with control mice. However, 4-week citalopram treatment altered the distribution of the number of lever presses in QNP-treated mice (Fig. 3D). Based on the devaluation index, 4-week administration of citalopram shifted goal-directed actions from QNP-induced habitual actions (Fig. 3E). There were no significant between-group differences in the consumption of both food pellets and sucrose solution during the free-feeding sessions (Fig. 3F). These results confirmed that repeated QNP injections did not result in goal-directed lever pressing; moreover, long-term SSRI treatment improved maladaptive habitual behaviour.
We then investigated whether PPI mitigates QNP-induced habitual behaviour (Fig. 3G). There was no significant between-group difference in the number of lever presses during training on an RR schedule (Fig. 3H). In the devaluation tests, vehicle or vonoprazan (3 μg) was injected 5 min before QNP injection. Further, the mice were fed food pellets on the valued day or sucrose solution on the devalued day freely in their home cage for 30 min. Each day, the mice underwent a 5-min lever pressing session immediately after the free access session. Lever presses on the valued or devalued day were recorded for the 2-day devaluation tests (Fig. 3I). In vehicle-injected QNP-treated mice, there was no statistically significant change in lever presses between valued or devalued condition. Contrastingly, vonoprazan injection restored the devaluation-induced reduction in lever presses (Fig. 3J). Consistently, vehicle-injected QNP-treated mice showed a significantly lower devaluation index than control mice, while vonoprazan increased the devaluation index in QNP-treated mice (Fig. 3K). In the free-feeding sessions, there was no significant between-group difference in the intake of pellets or sucrose solution (Fig. 3L). These results indicated that proton pump blockade could effectively ameliorate the QNP-induced habitual action.
PPI attenuated hyperactivity in the lateral orbitofrontal cortex (OFC) pyramidal neurons induced by repeated D2R stimulation
The results of the intracerebroventricular injection experiments demonstrated that the effects of PPIs resulted from their action on the brain. Patients with OCD exhibit hyperactivity in the OFC ameliorated only in SSRI-responsive patients [35, 36]. Therefore, we hypothesised that PPIs modulate neural activity in the OFC. Accordingly, we examined the effects of vonoprazan (100 mg/kg) on the expression of c-Fos, a neural activity marker, in the lateral OFC through immunostaining (Fig. 4A). QNP-treated mice showed an increased number of c-Fos-positive cells in the lateral OFC. In contrast, vonoprazan injection decreased the number of c-Fos-positive cells in QNP-treated mice (Fig. 4B, C).
Fig. 4: Administration of vonoprazan inhibited hyperactivity in lateral OFC pyramidal neurons obtained from QNP-treated mice.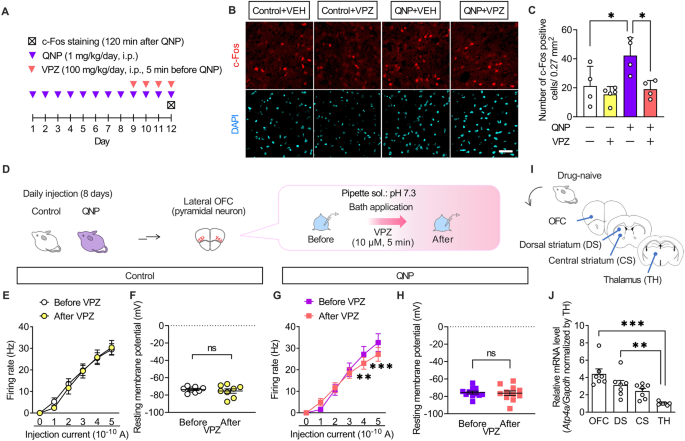
A Mice were intraperitoneally treated with QNP (1 mg/kg) for 8 days as well as the vehicle (VEH) or vonoprazan (VPZ; 100 mg/kg) 5 min before QNP injection on the 9th–12th day. Coronal sections containing the lateral OFC were prepared 120 min after the final QNP injection. B Representative images of c-Fos-positive cells in the lateral OFC. Red: c-Fos, a marker for activated neurons; Cyan: DAPI; Scale bar = 50 μm. C The number of c-Fos-positive cells in the lateral OFC. D–H The firing responses induced by current injection (0–500 pA; 1 s duration) were recorded before and after bath application of VPZ (10 μM) in intracellular pH 7.3 in the lateral OFC pyramidal neurons obtained from control (E, F) and QNP-treated mice (G, H). E, G Firing responses induced by current injection. F, H The resting membrane potential. I Samples containing brain regions of the cortico-striato-thalamo-cortical circuit were obtained from drug-naive mice. J Relative Atp4a mRNA levels in each brain region. ns: P > 0.05; *P P P
Further, we examined the effects of vonoprazan on the neural activity of lateral OFC pyramidal neurons using ex vivo patch-clamp recordings (Fig. 4D). Bath vonoprazan application (10 μM) did not affect the current-induced firing responses in lateral OFC pyramidal neurons compared with those in control mice (Fig. 4E, F). However, these were significantly decreased by vonoprazan treatment in QNP-treated mice (Fig. 4G, H). The inhibiting effects of vonoprazan on firing response were prominent in the late phase of the depolarising pulse (Supplementary Fig. 2).
The expression and function of P-type proton pumps in the brain remain unclear. We examined the expression pattern of Atp4a mRNA, which encodes the catalytic subunit of the P-type proton pump, to identify the brain regions involved in the effects of PPIs [37]. As patients with OCD have altered functional connectivity within the CSTC-loop [38, 39], we analysed mRNA expression levels of Atp4a in the CSTC-loop-related brain regions through real-time qRT-PCR (Fig. 4I). In drug-naive mice, Atp4a mRNA expression levels were higher in the OFC than in the thalamus (Fig. 4J). These results demonstrated that vonoprazan decreased neuronal hyperactivity in the lateral OFC of QNP-treated mice by inhibiting P-type proton pumps.
PPI attenuated neuronal activity through intracellular acidification of primary cultured cortex neurons
Vonoprazan inhibits acid release into the extracellular environment through P-type proton pumps and may increase intracellular proton concentration [26]. Accordingly, we visualised the intracellular pH of primary cultured mouse cortical neurons using super-ecliptic pHluorin (SEpHluorin) [40,41,42], which is a pH-sensitive fluorescence protein that shows reduced fluorescence intensity under an acidic environment (Fig. 5A). Vonoprazan (10 μM) significantly decreased the intracellular pH compared with the vehicle (Fig. 5B).
Fig. 5: Administration of vonoprazan inhibited neuronal activity through intracellular acidification.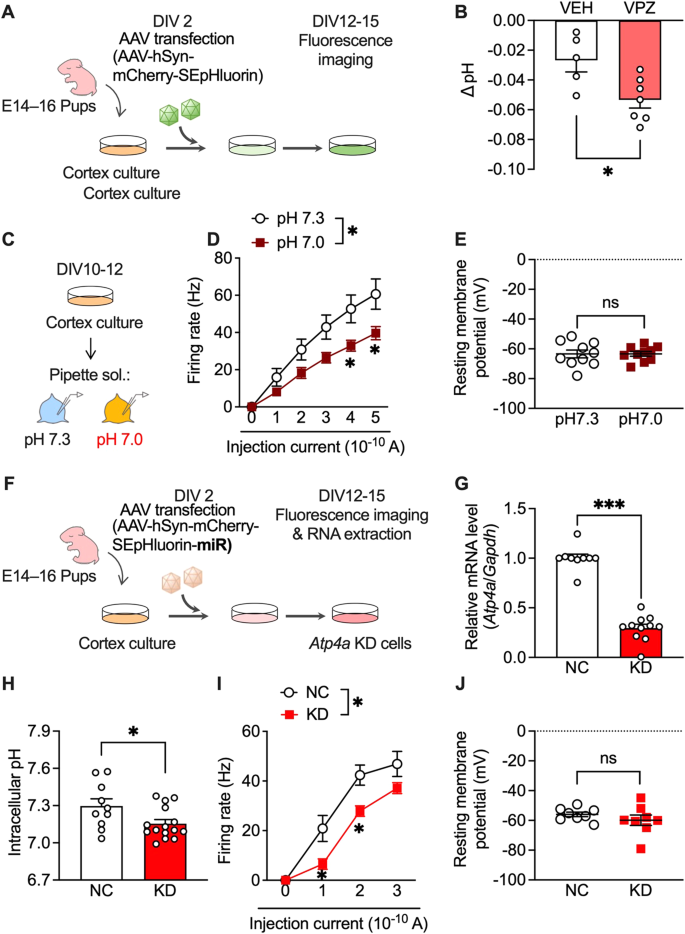
A AAV-infected SEpHluorin-expressing primary cultures of mouse cortex neurons. B Intracellular pH at 5 min after vehicle (VEH) application or vonoprazan (VPZ; 10 μM). C–E Electrophysiological recordings of naive primary cultures of mouse cortex neurons in intracellular pH 7.3 or 7.0. D Firing responses induced by current injection (0–500 pA; 1 s duration). E The resting membrane potential. F–J AAV-SEpHluorin-miRNA-mediated negative control (NC) or Atp4a KD in primary cultures of mouse cortex neurons. G Relative expression levels of Atp4a mRNAs in cortex cultures at 12 days after AAV infection. H Intracellular pH of AAV-infected neuronal cultures. I, J Electrophysiological recordings of Atp4a KD primary cortex neurons. Firing activity elicited by current injection (I) and the resting membrane potential (J) is shown. ns: P > 0.05; *P P
Additionally, we examined the effects of intracellular acidification on neural activity in cultured cortical neurons. Firing responses induced by the depolarising current were recorded in normal (pH 7.3) and slightly acidic (pH 7.0) pipette solutions (Fig. 5C). The firing rate was significantly lower under acidic conditions than under normal conditions (Fig. 5D). There was no significant between-group difference in resting membrane potentials (Fig. 5E). These results showed that PPI-mediated intracellular acidification contributes to the inhibitory effects in lateral OFC neurons.
Next, we investigated whether P-type proton pumps in cortical neurons are crucial in controlling intracellular pH and neuronal activity. Atp4a knockdown (KD) was achieved using miRNA-encoding AAV (Fig. 5F), which significantly decreased Atp4a mRNA expression compared with AAV encoding the negative control (NC) miRNA (Fig. 5G). Consistent with vonoprazan experiment results (Fig. 5B), neuron-specific Atp4a KD significantly decreased the intracellular pH (Fig. 5H) and significantly inhibited the current-induced firing responses without altering the resting membrane potential (Fig. 5I, J).
PPI attenuated neuronal activity under acidic intracellular conditions in lateral OFC pyramidal neurons
P-type proton pumps in cortical neurons are involved in the homoeostatic maintenance of intracellular pH and membrane excitability modulation. We recorded firing activity under slightly acidic intracellular conditions (pH 7.0) to confirm whether the inhibitory effects of vonoprazan on neuronal activity were facilitated by weak intracellular acidosis (Fig. 6A). At pH 7.0, vonoprazan significantly decreased the firing activity of lateral OFC pyramidal neurons in control mice (Fig. 6B, C). Vonoprazan significantly decreased the firing activity in the late phase of the depolarising pulse (Supplementary Fig. 2). These inhibitory effects of vonoprazan were similarly observed in QNP-treated mice. Additionally, there was significant inhibition at lower injection currents (200 and 300 pA) than in normal pH conditions (Fig. 6D, E). In control and QNP-treated mice, the combination of intracellular acidic conditions and vonoprazan treatment caused slight but significant hyperpolarisation of the resting membrane potential. These results further indicated that vonoprazan inhibited the firing activity of lateral OFC pyramidal neurons through intracellular proton accumulation.
Fig. 6: Vonoprazan attenuated neuronal activity in the lateral OFC under acidic intracellular conditions.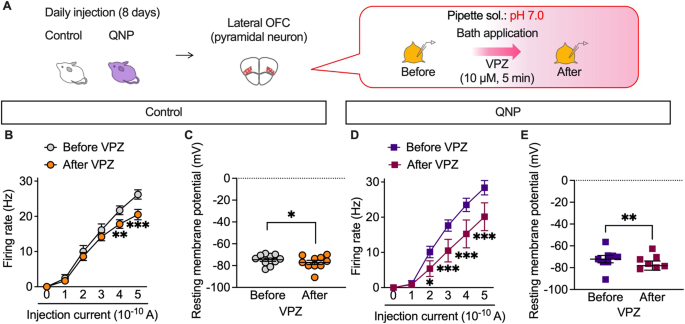
A–E The firing responses induced by current injection (0–500 pA; 1 s duration) were recorded before and after bath application of vonoprazan (VPZ; 10 μM) in intracellular pH 7.0 in the lateral OFC pyramidal neurons obtained from control (B, C) and QNP-treated mice (D, E). B, D Firing responses induced by current injection; C, E the resting membrane potential. *P P P
The preclinical experiment results supported the data-driven hypothesis that PPIs inhibited OCD-like symptoms.