Introduction
According to WHO 2018, worldwide obesity has nearly tripled since 1975 (http://www.who.int/mediacentre/factsheets/fs311/en/). Orlistat was the only available weight-loss medicine since 1999. Recently, four new anti-obesity drugs (lorcaserin, phentermine/topiramate, altrexone/bupropion and liraglutide 3.0 mg) for long-term use were approved in the USA. The new drugs were clinically effective, but their high price and risk of adverse effects shouldn’t be ignored (1). Thus, new candidate drugs are still urgently needed.
Memantine hydrochloride, an uncompetitive N-methyl-D-aspartate receptor (NMDAR) antagonist, was approved in Europe (in 2002, marketed under the product names Ebixa and Axura) and the US (in 2003, marketed as Namenda) for moderate-to-severe Alzheimer’s disease (AD) (2). Memantine is not only used for neurodegenerative diseases, but also for some neuropsychiatric syndromes, like binge eating disorder. Several studies have shown that memantine can significantly correct the binge-like eating behavior in human and animal models (3–6). As we know, our eating behavior can decide our whole day caloric intake. Eating behavior plays important role in obesity by modulating hormones such as letpin and ghrelin, which are related to BMI and body fat (7, 8). The imbalance of leptin and ghrelin affects the brain rewards system and promotes overeating (7, 8). People with overeating behavior are more likely to be obese (9–12). Therefore, we suppose that memantine would be a good candidate for the treatment of obesity. Further studies on whether and how memantine increases weight loss are needed.
The present study aimed to investigate the effects of memantine on weight loss. By taking advantage of the obesity mouse model, we firstly explored whether long-term NMDAR antagonism by memantine could systemically increase weight loss, and then tried to explain potential mechanisms.
Materials and Methods
Animals
Male C57BL/6J mice were housed in standard cages (48 cm × 26 cm), with controlled temperature (22°C) and a 12 h light/12 h dark cycle. There were four mice in each cage. All procedures were carried out in accordance with the guidelines for the Care and Use of Laboratory Animals of Shanghai Jiao Tong University School of Medicine and approved by the Institutional Animal Care and Use Committee [Department of Laboratory Animal Science (DLAS), Shanghai Jiao Tong University School of Medicine] (Policy Number DLAS-MP-ANIM.01–05).
Long-Term NMDAR Antagonism by Memantine on Obesity
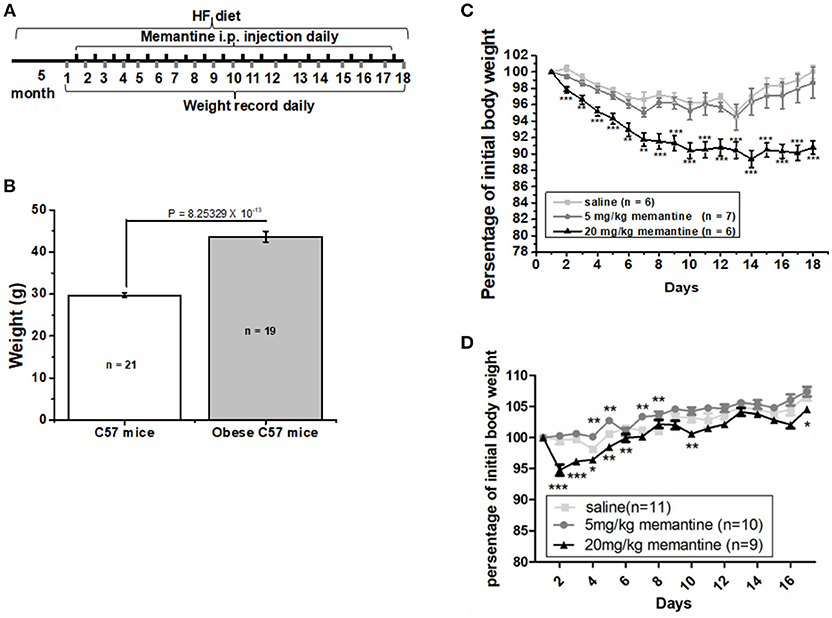
Six-week old C57BL/6J male mice were fed with high fat food (HF group) and standard control food (Ctrl group) for 5 months. The procedure was carried out as illustrated in Figure 1A. The high fat food (HF) contained a total of 45% kcal from fat (D12451, Research Diets, Inc., USA). At the end of the 5 months, the weight of mice in both groups was recorded. Then obese mice were divided into three groups, which were given saline, 5 and 20 mg/kg memantine, respectively. Memantine (Sigma, USA) dissolved in 0.9% saline was injected intraperitoneally for 17 days. During these days, the obese mice were continually fed with HF food. In order to investigate the effects of memantine on C57 mice fed with standard control food, memantine with different doses was also injected intraperitoneally. The body weight during the 17 days was recorded daily.
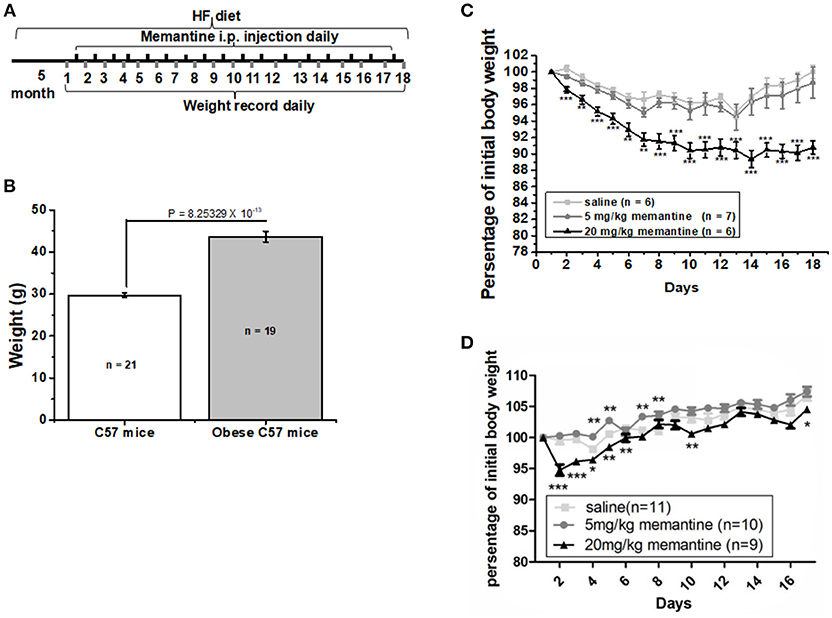
Figure 1. Long-term NMDAR antagonist treatment by memantine decreased the weight of obese mice. (A) Schematic representation for memantine injection and weight recording. (B) The weight of HF food diet group mice was significantly larger than that of Ctrl food diet group mice (unpaired Student’s t-test, p = 8.25329 × 10−13). (C) Memantine significantly decreased the percentage of body weight to original body weight during memantine injection days [Day, F(17, 342) = 5.577, P = 0.000; Group, F(2, 342) = 107.155, P = 0.000; Day*Group, F(51, 342) = 1.375, P = 0.058, two way ANOVA; from the second day to the end, all P P3 day = 0.0028; P6 day = 0.0023; P7 day = 0.0027, unpaired Student’s t-test; (D) The percentage of body weight in mice fed with standard control food diet and administered with 20 mg/kg memantine showed a significant decrease during the first memantine injection days but did not differ in late injection days (Day, F(15, 480) = 33.133, P = 0.000; Group, F(3, 480) = 73.964, P = 0.000; Day*Group, F(51, 342) = 1.429, P = 0.041, two way ANOVA). *P P P
Food Intake Test
Eight-week old C57BL/6J male mice were made to fast for 24 h as previously described (13). The procedure was carried out as illustrated in Figure 2A. Then they were habituated to the test box (mouse cage with new material) for 20 min. After habituation, saline and memantine (5 and 20 mg/kg) were intraperitoneally injected in the home cage. Thirty minute later, standard food was presented in the test box and the food that was consumed during the next 20 min was recorded.
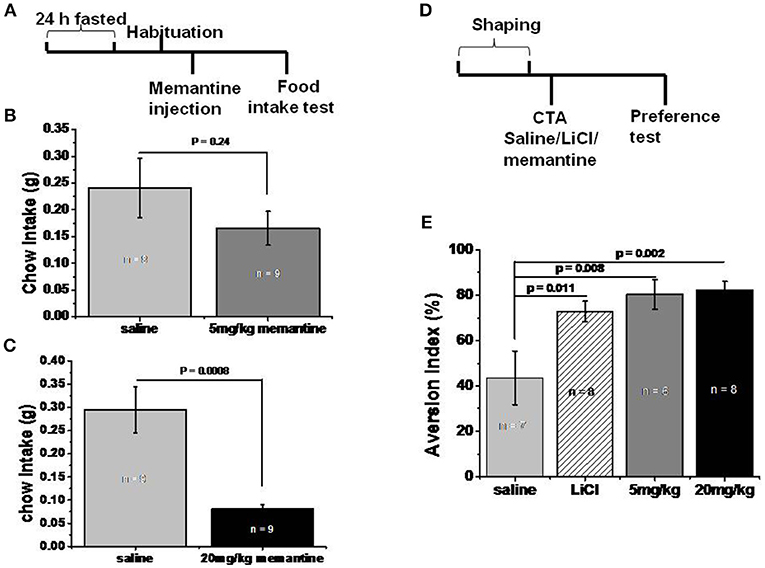
Figure 2. Memantine decreased food intake. (A) Schematic representation of standard food intake test protocol by memantine. (B) 5 mg/kg memantine group showed similar Ctrl food intake with saline group (unpaired Student’s t-test, P = 0.241). (C) 20 mg/kg memantine group significantly reduced the Ctrl food intake compared to saline group (unpaired Student’s t-test, P = 0.0008). (D) Schematic representation of CTA behavioral protocol. (E) LiCl group and memantine group decreased the intake of sodium saccharin solution [group, F(3, 28) = 4.707, P = 0.01, one way ANOVA], post-hoc analysis revealed LiCl, 5 and 20 mg/kg memantine group increased the aversion index to 72.88% (P = 0.011), 74.43% (P = 0.008), and 82.29% (P = 0.002) respectively, compared to 43.63% of saline group.
Conditioned Taste Aversion (CTA) Test
The CTA tests were performed as described previously with some modifications (14). The procedure was carried out as illustrated in Figure 2D. During the 1-week adaptation, 8-week old C57BL/6J male mice drank water once a day from two bottles (from 9:00 to 9:30 a.m.), but had free access to the standard control food. Water intake was recorded for each mouse by weighing both bottles before and after drinking time. Following the adaptation, each mouse was allowed to drink two bottles of 0.5% sodium saccharin solution (0.5% w/v) (Sigma-Aldrich) during the 30 min drinking time. Forty minute after drinking time, mice were given an intraperitoneal injection of saline, LiCl (0.15 M, Sigma-Aldrich), and memantine (5 and 20 mg/kg), respectively. On the day of the test, one bottle of 0.5% sodium saccharin solution and one bottle of water were inserted into each cage simultaneously. Fluid consumption was determined by weighting both bottles before and after drinking time. Aversion index (in %) = water intake (in grams) × 100%/[sodium saccharin intake (in grams) + water intake (in grams)].
Open Field Test
The test was carried out as previously described (15), in a square plexiglass apparatus (40 × 40 × 40 cm). A digital camera was set above the apparatus. Trace was recorded by the Ethovision video tracking system (Noldus Information Technology, Wageningen, Netherlands). Thirty minute before the test, 9-week old C57BL/6J male mice were intraperitoneally injected with saline and memantine (5 and 20 mg/kg). The mice were then gently placed in the apparatus and were left free to explore for another 60 min. After each trial, the apparatus was cleaned with 75% ethanol. In another 30 min test recorded by Tru Scan system (CoulBourn Instrument, USA), Cholecystokinin (CCK, 30 μg/kg, Tocris Bioscience, USA) and LiCl (150 mg/kg, Sigma-Aldrich, USA) were dissolved in saline and injected intraperitoneally 30 min before test.
Elevated Plus Maze Test
The protocols were followed as previously described (15). The black plastic elevated plus maze consisted of four 30 cm × 5 cm arms (two open without walls and two enclosed by 15.25 cm high walls). The maze was elevated 40 cm above the floor. Activity was recorded with a digital camera suspended from the ceiling. The test took place during the light phase. On the test day, mice were placed individually in the center of the maze facing the enclosed arms, and recorded for 5 min by the Ethovision tracking system (Noldus Information Technology, Wageningen, Netherlands). The maze was cleaned with 75% alcohol between trials. The time spent in the four arms was analyzed.
Statistical Analysis
Values were expressed as the mean ±S.E.M. Groups were compared using Student’ s t-test or ANOVA. P
Results
Memantine Increased the Weight Loss of Obese Mice
After 5 months, the weight of the HF food diet group was significantly larger than that of the Ctrl food diet group (Figure 1B). To investigate the effects of memantine on obesity, memantine was administered intraperitoneally. The percentage of body weight during the 17 memantine injection days compared to the original body weight was analyzed. Compared to the saline group mice, the percentage of body weight in mice fed with HF food diet and administered with 20 mg/kg memantine showed a significant decrease during the memantine injection days (Figure 1C). Meanwhile, the percentage of body weight for 5 mg/kg memantine group mice was similar to that of the saline group mice (Figure 1C). Compared to the saline group mice, the percentage of body weight in mice fed with standard control food diet and administered with 20 mg/kg memantine showed a significant decrease during the early memantine injection days but did not differ in later injection days (Figure 1D). By contrast, the percentage of body weight in mice administered with 5 mg/kg memantine injection was similar with that of saline group mice, while higher in some days (Figure 1D). These data showed the potential of memantine on obesity control.
Memantine Decreased Food Intake
In order to investigate whether memantine decreased the weight of obese mice by decreasing food intake, mice that fasted for 24 h were intraperitoneally injected with saline and memantine (5 and 20 mg/kg). The 5 mg/kg memantine group showed similar Ctrl food intake to the saline group (Figure 2B). However, the 20 mg/kg memantine group significantly reduced the Ctrl food intake compared to the saline group (Figure 2C). In order to find out whether memantine leads to severe side effect, like abdominal discomfort, CTA model was used. Compared to the saline group, the LiCl, and memantine groups saw a significant decrease in the intake of sodium saccharin solution (Figure 2E).
Memantine Increased Locomotor Activity Without Severe Side Effect
Open field test was performed to clarify whether memantine decreased food intake due to abdominal discomfort. Both 5 and 20 mg/kg memantine mice groups showed significantly increased locomotor activity compared to the saline mice group (Figure 3A). Memantine wasn’t found to induce anxiety because mice injected with memantine spent more time in the center of the open field than the control group mice (Figure 3B). Besides, in the elevated plus maze test, mice injected with memantine spent similar time in the open arms compared to control group mice (Figure 3C). In order to explore the behavior under satiation and abdominal discomfort condition, CCK and LiCl were used. LiCl group mice covered significant less distance during the time in open field (Figure 3D), while CCK group mice showed a behavior similar to the saline group mice. These results suggest that no abdominal discomfort and no anxiety are induced by memantine.
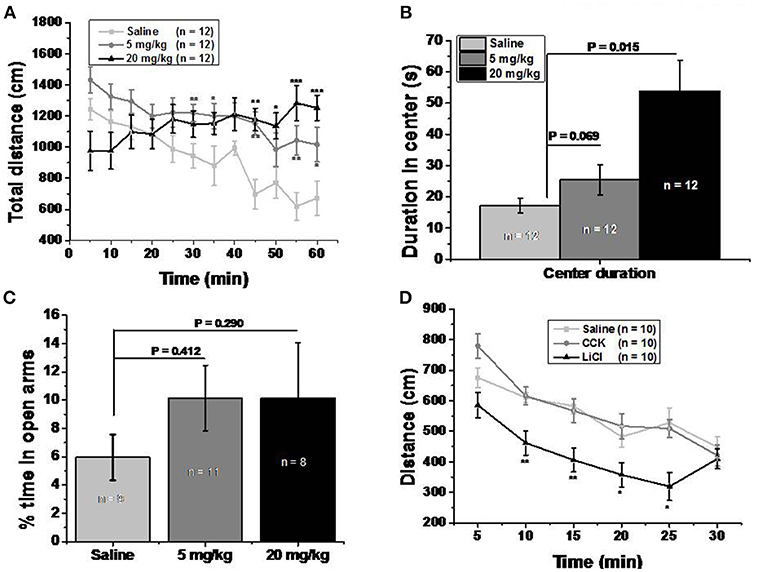
Figure 3. Memantine increased locomotor activity without severe side effect. (A) Both 5 and 20 mg/kg memantine group increased the distance during the late period of open field test. [Group, F(2, 360) = 26.906, P = 0.000; Time, F(11, 360) = 2.543, P = 0.004; Group*Time, F(22, 360) = 2.810, P = 0.000, two way ANOVA; saline group and 5 mg/kg memantine group, P30 = 0.009, P35 = 0.047, P45 = 0.001, P55 = 0.004, P60 = 0.042; saline group and 20 mg/kg memantine group, P55 = 0.001, P50 = 0.02, P55 = 0.0004, P60 = 0.0008, unpaired Student’s t-test; *P P P (B) Mice injected with memantine spent more time in the center of open field than that of control group mice [group, F(2, 29) = 8.652, P = 0.001, one way ANOVA; post-hoc analysis showed that saline group and 5 mg/kg memantine group, P = 0.069; saline group and 20 mg/kg memantine group, P = 0.015]. (C) Mice injected with memantine spent similar time in open arms with that of saline group mice [group, F(2, 27) = 0.64, p = 0.536, one way ANOVA; post-hoc analysis saline group and 5 mg/kg memantine group, P = 0.412; saline group and 20 mg/kg memantine group, P = 0.290]. (D) LiCl group mice showed significant less distance during the time in open field [group, F(2, 180) = 27.270, P = 0.000; time, F(5, 180) = 20.181, P = 0.000; group*time, F(10, 180) = 0.316; two way ANOVA; saline and LiCl group, P10 = 0.002, P15 = 0.002, P20 = 0.039, P25 = 0.012, unpaired Student’s t-test; (D)].
Discussion
Our results show that long term NMDAR antagonism by memantine significantly decreased the weight of obese mice. Our results are in accordance with clinical reports. By using an on-off-on design, Schaefer et al. found that memantine discontinuation and re-exposition were followed by a significant weight increase and a substantial weight loss (16). In a therapeutic trial in five obese women, Hermanussen et al. found that memantine could significantly suppress the appetite and binge-eating disorder and finally decrease the body weight within a few days (5). In our results, compared to the saline group mice, the percentage of body weight in mice fed with standard control food diet and administered with 20 mg/kg memantine showed a significant decrease during the early memantine injection days but was similar in later injection days. Meanwhile, the percentage of body weight in mice administered with 5 mg/kg memantine injection was similar with that of saline group mice, while higher in some days. There are few reports about whether memantine affects the weight of people with healthy weight. Under standard institutionalized diet, Venturelli et al. found that BMI decreased significantly in Alzheimer’s Disease (AD), while in CTRL it remained unchanged with similar levels of daily energy expenditure. The combination of three factors, number of medications taken, albuminemia, and cortisolism, predicted ΔBMI in Woman with AD (17). Several studies have reported NMDAR signaling in the regulation of appetite (18–22). NMDAR signaling regulates food intake at several appetite-suppressing nodes, including the solitary tract nucleus (23–25), the parabrachial nucleus (26, 27), the ventromedial nucleus of the hypothalamus and the paraventricular nucleus of the hypothalamus (22, 28), and the lateral habenula (29). In another study, the central amygdala (CeA) region was shown to play an important role in appetite regulation (13). Further research needs to be carried out to elucidate which brain areas are involved in the mechanism of memantine on obesity. Because of the important role of peptides (like leptin and ghrelin) in appetite related brain areas like hypothalamus, the expression of these peptides in brain may change.
Our results showed that memantine decreased the weight of obese mice by suppression of food intake. In the CTA model, memantine had similar effects to LiCl. Traverso et al. reported that MK-801, another NMDAR antagonist, induced low intensity conditioned taste aversion (30). MK-801 was reported to virtually block all NMDAR activity and manifested unacceptable side effects (31). Differently, memantine preferentially blocks excessive (pathological/ extrasynaptic) NMDAR activity and its activity remains mostly normal (physiological/synaptic) due to an uncompetitive mechanism of action in conjunction with a relatively fast off-rate, resulting in a low affinity for the NMDAR (31). Combined with our findings that memantine decreased food intake in CTA model, we suppose that the mechanism of memantine that suppresses food intake may be different from that of MK-801.
In the CTA model, our results showed that memantine had similar effect to LiCl. As we know, LiCl can induce abdominal discomfort. If memantine did induce abdominal discomfort, it would suppress the locomotor activity of mice in open field test. Interestingly, our results showed that LiCl group mice had less locomotor activity than saline group mice, while memantine didn’t suppress but increased the locomotor activity in open field test. And memantine wasn’t shown to cause anxiety. There are many studies about the effects of memantine on locomotion. When the time periods of open field test differ, the results can be different. In the 5 min open field test, the total distance traveled by rodents with and without memantine injection is similar (32). During the first 60 min open field test, Costa et al. found that the total distance increased following the increase of memantine doses (33). In the last 5 min of the 30 min open field test, Kotermanski et al. found that the total distance traveled by rats was increased following the increase of memantine doses (34). The duration of the period spent in the center of the open field could reflect the anxiety level in open field test, and the results among different studies differed. In the 5 min open field test, Camarasa et al. found that memantine-treated rats spent longer time in the center and shorter time in the periphery (35). When analyzed with different parameters or when the method of memantine intake differed, the anxiety level in the open field could differ (35, 36). Our results in plus maze showed that the percentage of duration in open arms (compared to duration in total arms) among different memantine groups was similar.
Foltin et al. reported that memantine decreased the food intake by enhancing the satiation (37). In our results, unlike LiCl suppressed locomotor activity, CCK group mice performed like saline group mice in open field test. We hypothesize that the decreased food intake and increased weight loss caused by memantine might be due to satiation.
Our results showed that memantine increased the locomotor activity in open field. It has been well known that exercise can improve health. In an elegantly designed study, Ross et al. (38) reported that the diet-induced and exercise-induced weight loss groups showed approximately 8% weight reduction, and had significant reductions in total fat mass, visceral fat and increased glucose disposal. However, when compared to the diet induced weight loss group, exercise training induced weight loss group had a greater reduction in total fat mass (39). In the sixth century B.C., Susruta advocated exercise as a treatment for diabetes (40). Muscle contractions and exercise increase energy consumption, glucose uptake (41, 42) and sensitivity of muscle to insulin (43). Adipose tissue and liver are also targeted by exercise. Adipose tissue is an active endocrine organ (44) that is dramatically influenced by exercise (45). Similarly, the liver helps mediating the beneficial effects of exercise (46). So, increased locomotor activity by memantine might lead to improvements in glucose homeostasis and decreased markers of liver damage in obese mice. Further studies are needed. However, Zimmer et al. found that long-term administration of memantine could induce anxiety-like behavior (47).
Conclusion
Long term NMDAR antagonism by memantine increases weight loss in mice obesity induced by high fat diet. Memantine decreases food intake without inducing abdominal discomfort and anxiety, suggesting that this compound would be a good candidate drug for obesity control. However, the molecular mechanism and brain circuit involved in the regulation of weight loss by memantine need further study.
Author Contributions
W-GL and FL designed the study and modified the manuscript. S-ND conducted the study and prepared the manuscript. Y-HY helped conduct the weight loss experiments and modify the manuscript. T-LZ and B-KM helped perform food intake experiments. H-RF and Y-ML helped finish open field tests and plus maze test.
Funding
The work was supported by National Natural Science Foundation of China (Nos. 81701334, 81571031, 81761128035), Shanghai Municipal Commission of Health and Family Planning (Nos. 2017ZZ02026, 2018BR33, 2017EKHWYX-02, and GDEK201709), Shanghai Shenkang Hospital Development Center (No. 16CR2025B), Shanghai Municipal Education Commission (No. 20152234), Shanghai Committee of Science and Technology (Nos. 17XD1403200, 18DZ2313505, and 18QA1402500), Xinhua Hospital of Shanghai Jiao Tong University School of Medicine (2018YJRC03, Talent introduction-014, Top talent-201603).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Tian-Le Xu and Mrs. Xiao-Ting Zhu for their kind support.
References
2. Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–too little activation is bad, too much is even worse. Neuropharmacology (2007) 53:699–723. doi: 10.1016/j.neuropharm.2007.07.013
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Popik P, Kos T, Zhang Y, Bisaga A. Memantine reduces consumption of highly palatable food in a rat model of binge eating. Amino Acids (2011) 40:477–85. doi: 10.1007/s00726-010-0659-3
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Smith KL, Rao RR, Velazquez-Sanchez C, Valenza M, Giuliano C, Everitt BJ, et al. The uncompetitive N-methyl-D-aspartate antagonist memantine reduces binge-like eating, food-seeking behavior, and compulsive eating: role of the nucleus accumbens shell. Neuropsychopharmacology (2015) 40:1163–71. doi: 10.1038/npp.2014.299
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Hermanussen M, Tresguerres JA. A new anti-obesity drug treatment: first clinical evidence that, antagonising glutamate-gated Ca2+ ion channels with memantine normalises binge-eating disorders. Econo Human Biol. (2005) 3:329–37. doi: 10.1016/j.ehb.2005.04.001
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Monteleone P and Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology (2013) 38:312–30. doi: 10.1016/j.psyneuen.2012.10.021
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin and resistin. Clin. Chem. (2004) 50:1511–25. doi: 10.1373/clinchem.2004.032482
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Jasinska AJ, Yasuda M, Burant CF, Gregor N, Khatri S, Sweet M, et al. Impulsivity and inhibitory control defcits are associated with unhealthy eating in young adults. Appetite (2012) 59:738–47. doi: 10.1016/j.appet.2012.08.001
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Guerrieri R, Nederkoorn C, Jansen A. The interaction between impulsivity and a varied food environment: its in?uence on food intake and overweight. Int J Obes. (2008) 32:708–14. doi: 10.1038/sj.ijo.0803770
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: Te role of impulsivity. Eat Behav. (2006) 7:315–22. doi: 10.1016/j.eatbeh.2005.11.005
CrossRef Full Text | Google Scholar
13. Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. (2014) 17:1240–8. doi: 10.1038/nn.3767
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Li WG, Liu MG, Deng S, Liu YM, Shang L, Ding J, et al. ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion. Nat Commun. (2016) 7:13770. doi: 10.1038/ncomms13770
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Deng S, Zhang L, Zhu T, Liu YM, Zhang H, Shen Y, et al. A behavioral defect of temporal association memory in mice that partly lack dopamine reuptake transporter. Sci Rep. (2015) 5:17461. doi: 10.1038/srep17461
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Schaefer M, Leopold K, Hinzpeter A, Heinz A, Krebs M. Memantine-associated reversal of clozapine-induced weight gain. Pharmacopsychiatry (2007) 40:149–51. doi: 10.1055/s-2007-984391
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Venturelli M, Cè E, Limonta E, Muti E, Scarsini R, Brasioli A, et al. Possible Predictors of involuntary weight loss in patients with Alzheimer’s disease. PLoS ONE (2016) 11:e0157384. doi: 10.1371/journal.pone.0157384
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. NMDA NR2 receptors participate in CCK-induced reduction of food intake and hindbrain neuronal activation. Brain Res. (2009) 1266:37–44. doi: 10.1016/j.brainres.2009.02.003
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Ritter RC. A tale of two endings: modulation of satiation by NMDA receptors on or near central and peripheral vagal afferent terminals. Physiol Behav. (2011) 105:94–9. doi: 10.1016/j.physbeh.2011.02.042
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Shoham S, Javitt DC, Heresco-Levy U. Chronic high-dose glycine nutrition: effects on rat brain cell morphology. Biol Psychiatry (2001) 49:876–85. doi: 10.1016/S0006-3223(00)01046-5
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Sorrels TL, Bostock E. Induction of feeding by 7-chlorokynurenic acid, a strychnine-insensitive glycine binding site antagonist. Brain Res. (1992) 572:265–8. doi: 10.1016/0006-8993(92)90481-N
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Tejas-Juarez JG, Cruz-Martinez AM, Lopez-Alonso VE, Garcia-Iglesias B, Mancilla-Diaz JM, Floran-Garduno B, et al. Stimulation of dopamine D4 receptors in the paraventricular nucleus of the hypothalamus of male rats induces hyperphagia: involvement of glutamate. Physiol Behav. (2014) 133:272–81. doi: 10.1016/j.physbeh.2014.04.040
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Campos CA, Ritter RC. NMDA-type glutamate receptors participate in reduction of food intake following hindbrain melanocortin receptor activation. Am J Physiol Regul Integr Comp Physiol. (2015) 308:R1–9. doi: 10.1152/ajpregu.00388.2014
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. Blockade of hindbrain NMDA receptors containing NR2 subunits increases sucrose intake. Am J Physiol Regul Integr Comp Physiol. (2009) 296:R921–8. doi: 10.1152/ajpregu.90456.2008
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Wright J, Campos C, Herzog T, Covasa M, Czaja K, Ritter RC. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Integr Comp Physiol. (2011) 301:R448–55. doi: 10.1152/ajpregu.00026.2011
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci USA. (2013) 110:14765–70. doi: 10.1073/pnas.1314137110
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Resch JM, Maunze B, Phillips KA, Choi S. Inhibition of food intake by PACAP in the hypothalamic ventromedial nuclei is mediated by NMDA receptors. Physiol Behav. (2014) 133:230–5. doi: 10.1016/j.physbeh.2014.05.029
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci. (2016) 36:302–11. doi: 10.1523/JNEUROSCI.1202-15.2016
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Nakamura T, Lipton SA. Preventing Ca2+−mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies. Cell Calcium. (2010) 47:190–7. doi: 10.1016/j.ceca.2009.12.009
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Borre Y, Bosman E, Lemstra S, Westphal KG, Olivier B, Oosting RS. Memantine partly rescues behavioral and cognitive deficits in an animal model of neurodegeneration. Neuropharmacology (2012) 62:2010–7. doi: 10.1016/j.neuropharm.2011.12.034
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Costa ACS, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of down syndrome on a fear conditioning test. Neuropsychopharmacology (2008) 33:1624–32. doi: 10.1038/sj.npp.1301535
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Kotermanski SE, Johnson JW, Thiels E. Comparison of behavioral effects of the NMDA receptor channel blockers memantine and ketamine in rats. Pharmacol Biochem Behav. (2013) 109:67–76. doi: 10.1016/j.pbb.2013.05.005
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Camarasa J, Rodrigo T, Pubill D, Escubedo E. Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacol Res. (2010) 62:450–6. doi: 10.1016/j.phrs.2010.05.004
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Rueda N, Llorens-Martín M, Flórez J, Valdizán E, Banerjee P, Trejo JL, et al. Memantine normalizes several phenotypic features in theTs65Dn mouse model of down syndrome. J Alzheimers Dis. (2010) 21:277–90. doi: 10.3233/JAD-2010-100240
CrossRef Full Text | Google Scholar
37. Foltin RW, Danysz W, Bisaga A. A novel procedure for assessing the effects of drugs on satiation in baboons: effects of memantine and dexfenfluramine. Psychopharmacology (2008) 199:583–92. doi: 10.1007/s00213-008-1178-8
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med. (2000) 133:92–103.
Google Scholar
39. Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. (2014) 56:441–7. doi: 10.1016/j.pcad.2013.09.012
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Holloszy JO, Narahara HT. Nitrate ions: potentiation of increased permeability to sugar associated with muscle contraction. Science (1967) 155:573–5. doi: 10.1126/science.155.3762.573
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol. (1988) 65:909–13.
PubMed Abstract | Google Scholar
43. Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. (1982) 69:785–93. doi: 10.1172/JCI110517
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. (2012) 92:157–91. doi: 10.1152/physrev.00012.2011
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Fealy CE, Haus JM, Solomon TP, Pagadala M, Flask CA, McCullough AJ, et al. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. J Appl Physiol. (2012) 113:1–6. doi: 10.1152/japplphysiol.00127.2012
PubMed Abstract | CrossRef Full Text | Google Scholar
47. Zimmer ER, Torrez VR, Kalinine E, Augustin MC, Zenki KC, Almeida RF, et al. Long-term NMDAR antagonism correlates reduced astrocytic glutamate uptake with anxiety-like phenotype. Front Cell Neurosci. (2015) 9:219. doi: 10.3389/fncel.2015.00219
PubMed Abstract | CrossRef Full Text | Google Scholar