1. Introduction
Adolescence is a critical stage in life, when healthy or risk behaviors of adulthood are first established, and social and emotional development affects decision-making and behavior (1, 2). The development of obesity or cardiovascular diseases can be accompanied by the emergence of mental health disorders such as depression, eating disorders, and low self-esteem (3, 4). Furthermore, oxidative stress has been linked with depression (5, 6), whereas a healthy dietary pattern is associated with good mental health (1, 7).
Obesity and metabolic syndrome (MS) are multifactorial diseases estimated to affect 5% of the global adolescent population in 2020 (8). MS is a cluster of risk factors for cardiovascular disease, type 2 diabetes, or prediabetes. These conditions include high blood pressure (BP), high triglycerides, and abdominal obesity, the latter being the key factor for MS diagnosis in adolescents (9). When these pathological triggers are present chronically, a dysfunctional mitochondria could be developed, which can cause an oxidative stress (10, 11), increasing its levels of biomarkers such as 8-isoprostane (12, 13). Isoprostanes are biosynthesized by a free radical-catalyzed peroxidation primarily from arachidonic acid, but also from docosahexaenoic and eicosapentaenoic acids (14, 15) and 8-isoprostane serves as a biomarker of oxidative stress status (16, 17). Due to the scant research on the relationship between 8-isoprostane and MS in adolescents, it is important to study this research topic and its association with factors such as lifestyle, emotion, and nutrition.
The aim of the present study was to analyze the association of nutritional parameters, lifestyle, emotion management, and MS with oxidative stress, measured by 8-isoprostane levels, in a cohort of adolescents enrolled in the SI! Program for Secondary Schools in Spain.
2. Materials and methods
The present cross-sectional analysis was performed in a sub-sample of participants enrolled at baseline (1st grade of Secondary School) in the SI! Program for Secondary Schools in Spain (clinical trials register: NCT03504059); all details have been previously published (18). A schematic view of the design and different phases of the present study is provided in Supplementary Figure S1.
2.1. Anthropometric measurements
Measurements were obtained after overnight fasting by trained nutritionists. Height was measured with a Seca 213 portable stadiometer (0.1 cm of precision). Body weight, body fat percentage, and skeletal muscle percentage were measured by bioelectrical impedance analysis (OMRON BF511 with 0.1 Kg precision), with participants wearing light clothes and no shoes. Body mass index (BMI) was calculated as body weight divided by height squared (kg/m2). Waist circumference (WC) was measured three times with a Holtain tape to the nearest 0.1 cm. Waist-to-height ratio (WHtR) was calculated as WC divided by height. BMI, WC, and WHtR were adjusted for age and sex to obtain z-score values (19, 20).
2.2. Biochemical analyzes
Biochemical blood analysis was performed by trained nurses using samples taken early in the morning after overnight fasting. Glucose, triglycerides, total cholesterol, high density lipoprotein cholesterol (HDL-c), and low density lipoprotein cholesterol (LDL-c) were determined in capillary blood samples using the Cardio Check Plus device and PTS Panels test strips (21). Fasting spot urine samples were collected in the morning. The urine was aliquoted and stored at −80°C for subsequent analysis. 8-Isoprostane concentration in urine was determined using the ELISA Kit protocol (Cayman Chemical, Ann Arbor, MI, United States, Item No. 516351). Creatinine was measured by the validated Jaffé alkaline picrate method (22). 8-Isoprostane levels were normalized by creatinine and the results were expressed as pg./mg creatinine.
2.3. Blood pressure
BP was measured with an OMRON M6 monitor with 2–3 min intervals between measurements. When the differences between the measurements were less than 10 mmHg for systolic blood pressure (SBP) and less than 5 mmHg for diastolic blood pressure (DBP), two measurements were taken; otherwise, a third reading was performed. Average values were calculated for the final SBP and DBP, which were adjusted for age, sex, and height to obtain percentile and z-score values (23, 24).
2.4. Physical activity and sleep characteristics
Moderate and vigorous physical activity levels and sleep duration were estimated from data from the triaxial accelerometer (Actigraph wGT3X-BT) worn on the non-dominant wrist for seven consecutive days. Activity information was considered valid if data were available for a minimum of 4 days, with at least 600 min of wear time per day. Physical activity intensities were estimated using the cut-off points of Chandler et al. (25). The sleep algorithm proposed by Sadeh et al. (26) was used to obtain sleep duration (total of hours asleep), sleep efficiency (number of sleep minutes divided by the total number of minutes the subject was in bed), awakenings (the number of awakenings per night), and time spent awake after initially falling asleep (the average length of all awakening episodes in minutes). All measurements were obtained using ActiLife software (Version 6.13.4, LLC).
2.5. Emotion management
To assess emotion management, three different subscales were used, namely “self-esteem,” “emotional eating” (EE), and “mood,” which were measured through validated questionnaires filled out by the participants. Four response categories on the Likert scale were used for self-esteem and EE items, and five response categories for mood items.
Self-esteem was assessed with five items of the Child Health and Illness Profile–Adolescent Edition test (CHIP-AE) (27). An example of an item is, “I like the way I am,” to which there are four response options: strongly disagree = 1, disagree = 2, agree = 3, and strongly agree = 4. Thus, higher scores designate better health-related outcomes. The score was obtained by calculating the mean scores. Internal reliability (Cronbach’s ⍺) was 0.797.
EE was assessed with three items of the Three-Factor Eating Questionnaire-R18 (Tfeq-Sp) (28). An example of an item is, “When I feel anxious, I find myself eating,” to which there are four response options: definitely false = 1, mostly false = 2, mostly true = 3, and definitely true = 4. Higher scores indicate greater levels of EE. Scores were categorized as “No EE,” “Low EE,” and “High EE.” The “No EE” category reflected a score of 3. To create the “Low EE” and “High EE” categories, a median split was used (excluding the “No” category), and scores below the median were categorized as “Low EE” and scores above the median as “High EE” (4). Internal reliability (Cronbach’s ⍺) was 0.791.
Mood was assessed with a six items of the validated FRESC (Factors de Risc en Estudiants de SeCundària) lifestyle risk-factor survey for secondary school students (29). An example of an item is, “I am too tired to do anything,” with the following response options: never, almost never, sometimes, frequently, and always. The variable was dichotomized, whereby the response “always” or “frequently” to three or more of the six items indicated a negative mood state, whereas a positive mood state was assigned for the other responses. Internal reliability (Cronbach’s ⍺) was 0.673 as reported by Ahonen et al. (30).
2.6. Dietary data
A validated semi-quantitative food frequency questionnaire with 157-items was filled out by the families of the participants to provide information about their dietary habits from the previous year (31, 32). The items were organized by food groups and the response categories were as follows: never or almost never, 1–3 per month, 1 per week, 2–4 per week, 5–6 per week, 1 per day, 2–3 per day, 4–6 per day, and 6 or more per day. This questionnaire results were analyzed using Evaldara software and the food composition tables of the Centro de Enseñanza Superior de Nutrición y Dietética and adjusted for total energy intake (33, 34).
2.7. Metabolic groups
The study population was categorized in three metabolic groups (healthy, at-risk, and MS) as defined by the International Diabetes Federation (9). Those in the MS group had abdominal obesity (≥ 90th percentile of WC) plus two or more clinical symptoms: ≥ 150 mg/dL of triglycerides or Supplementary Figure S2 shows a schematic view of the process followed in the different phases of defining the study groups.
2.8. Statistical analyzes
A minimum of 118 participants were required to provide 95% power of test and a significance level of 5% when performing multiple regression. Given that 44 participants presented MS, a total of 132 participants were enrolled to achieve a ratio of 1:1:1 for the three groups (44 in each); more details are provided in Supplementary Figure S2.
A seven-step statistical process was followed; a value of p ≤0.05 was considered statistically significant. First, a 98% winsorizing technique was used to minimize the influence of outliers. Second, numerical variables without prior standardization (i.e., adjusted for age, sex, and height) were standardized (z-scores) prior to the statistical analysis. Third, the normality of variables was determined by the Kolmogorov–Smirnov test. Fourth, to estimate differences, a chi-square test was used for categorical variables, an analysis of variance (ANOVA) for parametric numerical variables, and a Kruskal–Wallis test for non-parametric numerical variables, whereas Dunn–Bonferroni correction was used to ascertain differences between the metabolic groups. Fifth, a simple linear regression was used to identify the association between 8-isoprostane levels and each studied variable. Sixth, principal component analysis was used to eliminate collinearity among variables. Finally, to capture more complex interactions between different factors, a multiple linear regression model was generated. All statistical analyzes were conducted using R software version 4.1.0 (R Studio, 250 Northern Ave, Boston, MA, United States).
3. Results
The present study enrolled 132 adolescents (48.5% girls) aged 12 ± 0.48 years. The healthy group had the highest percentage of girls (56.8%), and the MS group the highest percentage of boys (63.6%) (Table 1).
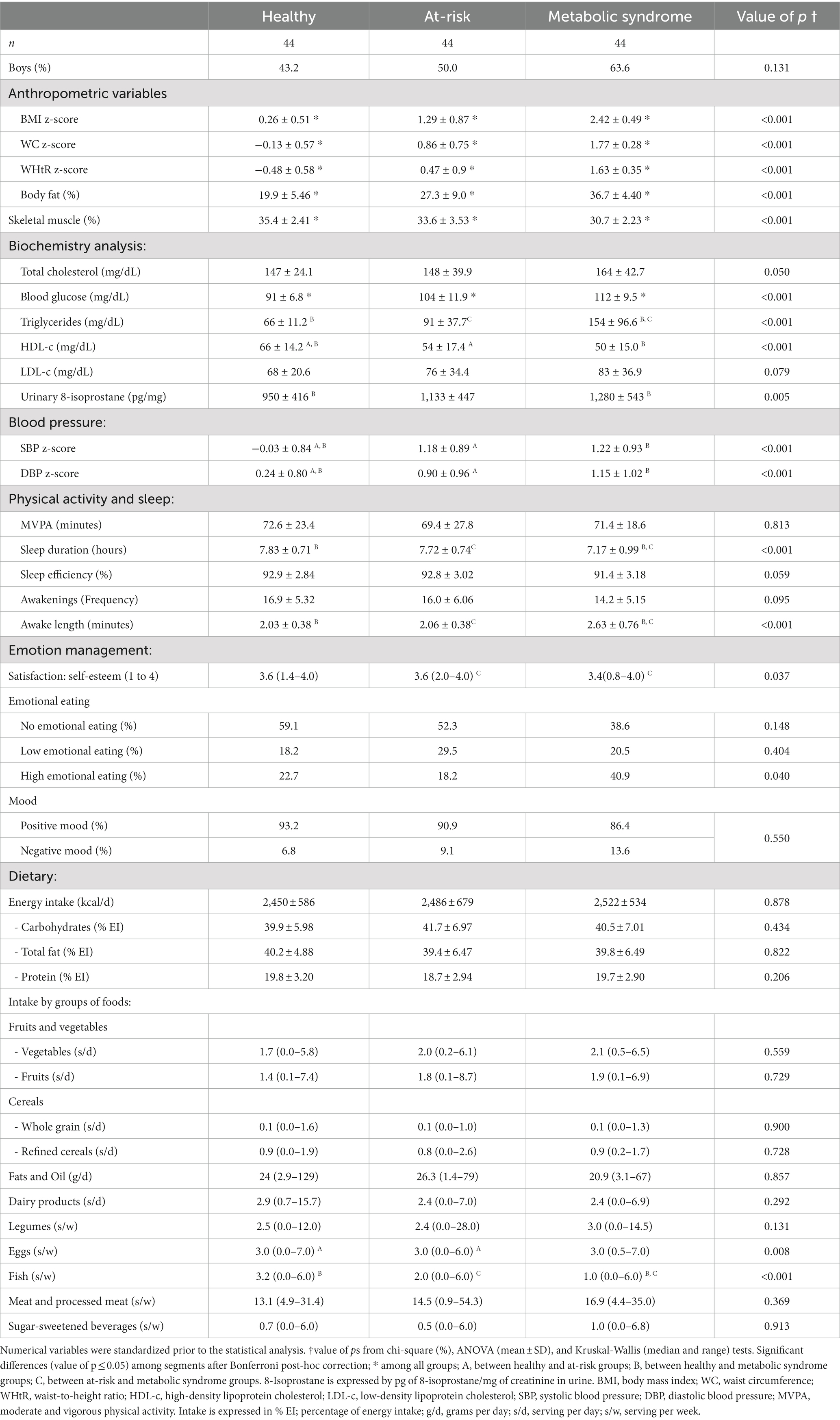
Table 1. Description and comparison of the metabolic groups.
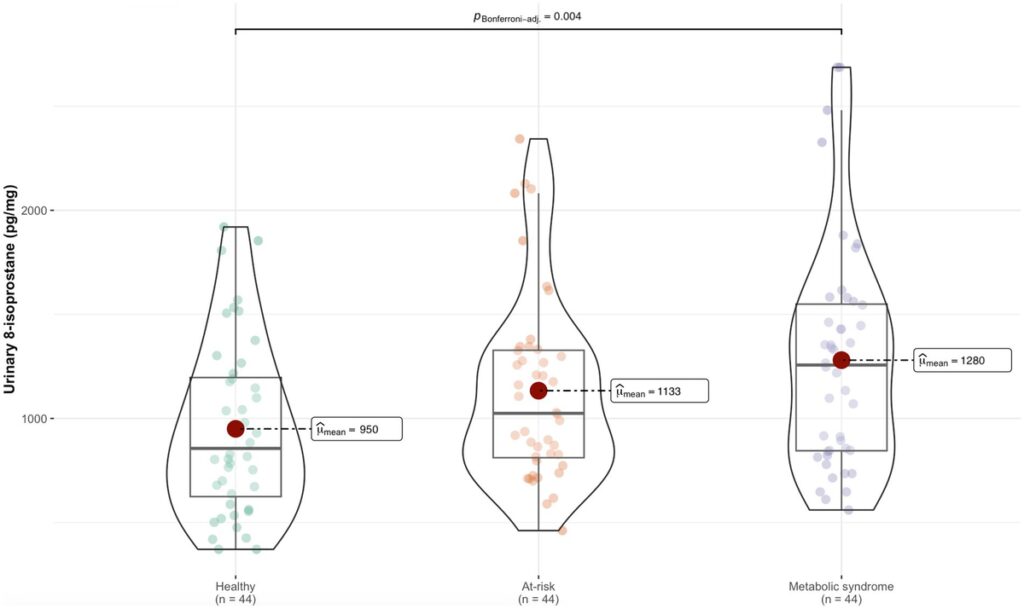
The characteristics of the study population and the differences between the three metabolic groups are described in Table 1 and Supplementary Table S1 shows the differences between sexes. Significant differences in all anthropometric measurements and blood glucose levels were observed for all metabolic groups. Triglycerides, HDL-c, 8-isoprostane levels, BP, sleep duration, time spent awake after initially falling asleep, and fish intake differed significantly between the healthy and MS groups. It is worth noting that the adolescents in the healthy group had longer periods of sleep interrupted by shorter awake periods compared to the MS group.
Levels of 8-isoprostane were higher in the MS group than the healthy group (Figure 1). Table 2 shows the association between 8-isoprostane and each nutritional parameter and lifestyle variable. The BMI, WC, and WHtR z-scores, and body fat percentage were positively associated with 8-isoprostane levels, suggesting the biomarker was positively related to adiposity. Similarly, blood glucose, total cholesterol, LDL-c, EE, categorization in the MS group, and refined cereal intake were positively associated with 8-isoprostane; in contrast, sleep duration and fish intake showed a negative association with 8-isoprostane (Table 2). Total cholesterol and z-scores for BMI, WC, and WHtR acted as collinear variables in the dataset under analysis.
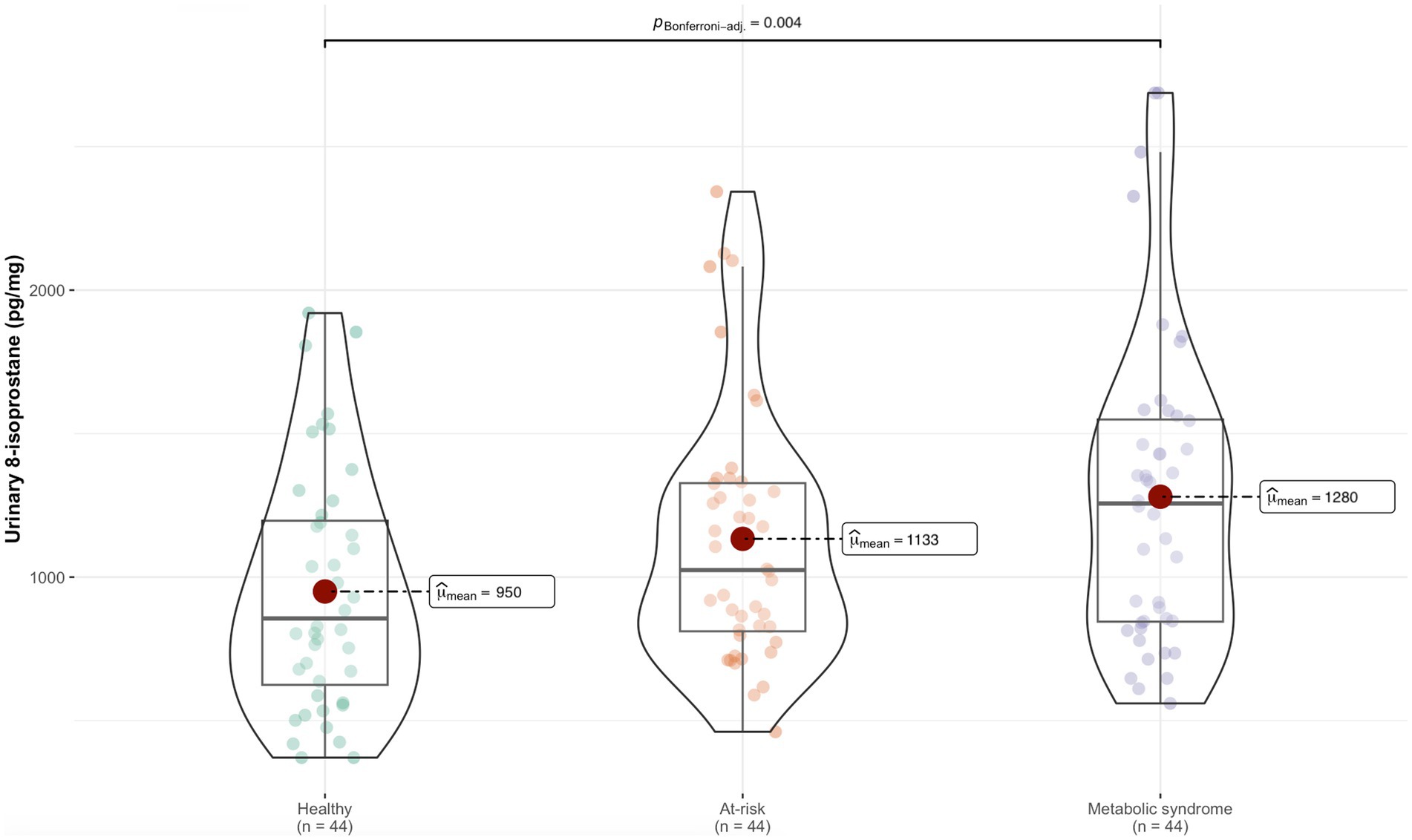
Figure 1. Difference in 8-isoprostane levels between the metabolic groups.
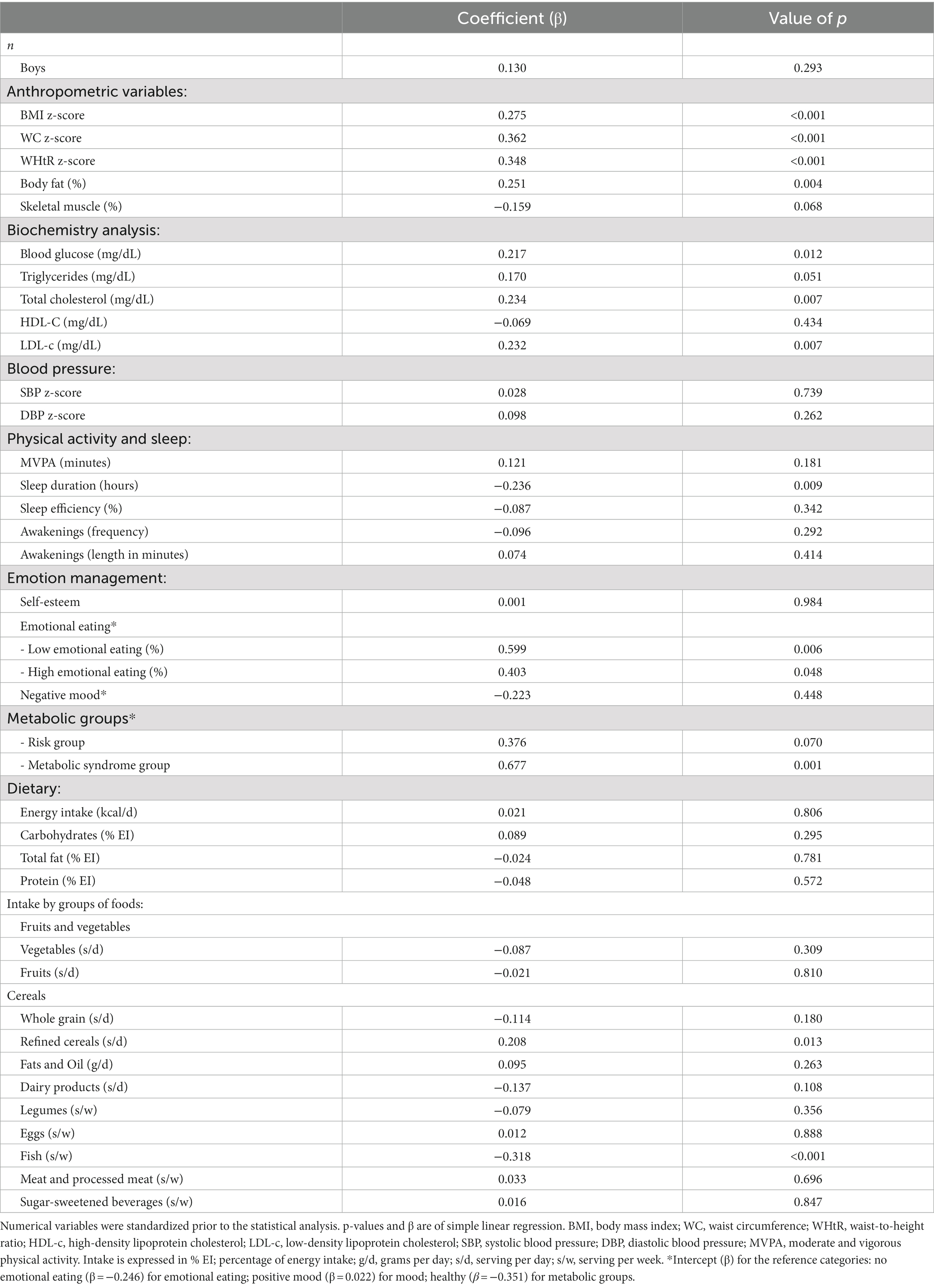
Table 2. Association of individual variables with 8-isoprostane.
Finally, the multiple adjusted linear regression model (Figure 2) showed an association between 8-isoprostane and metabolic group (at-risk β = 0.140, value of p = 0.513; MS β = 0.213, value of p = 0.470), blood glucose (β = 0.124, value of p = 0.247), LDL-c (β = 0.173, value of p = 0.049), sleep duration (β = −0.026, value of p = 0.798), EE (low β = 0.443, value of p = 0.036; high β = 0.151, value of p = 0.470), refined cereal intake (β =0.191, value of p = 0.024), and fish intake (β = −0.187, value of p = 0.050).
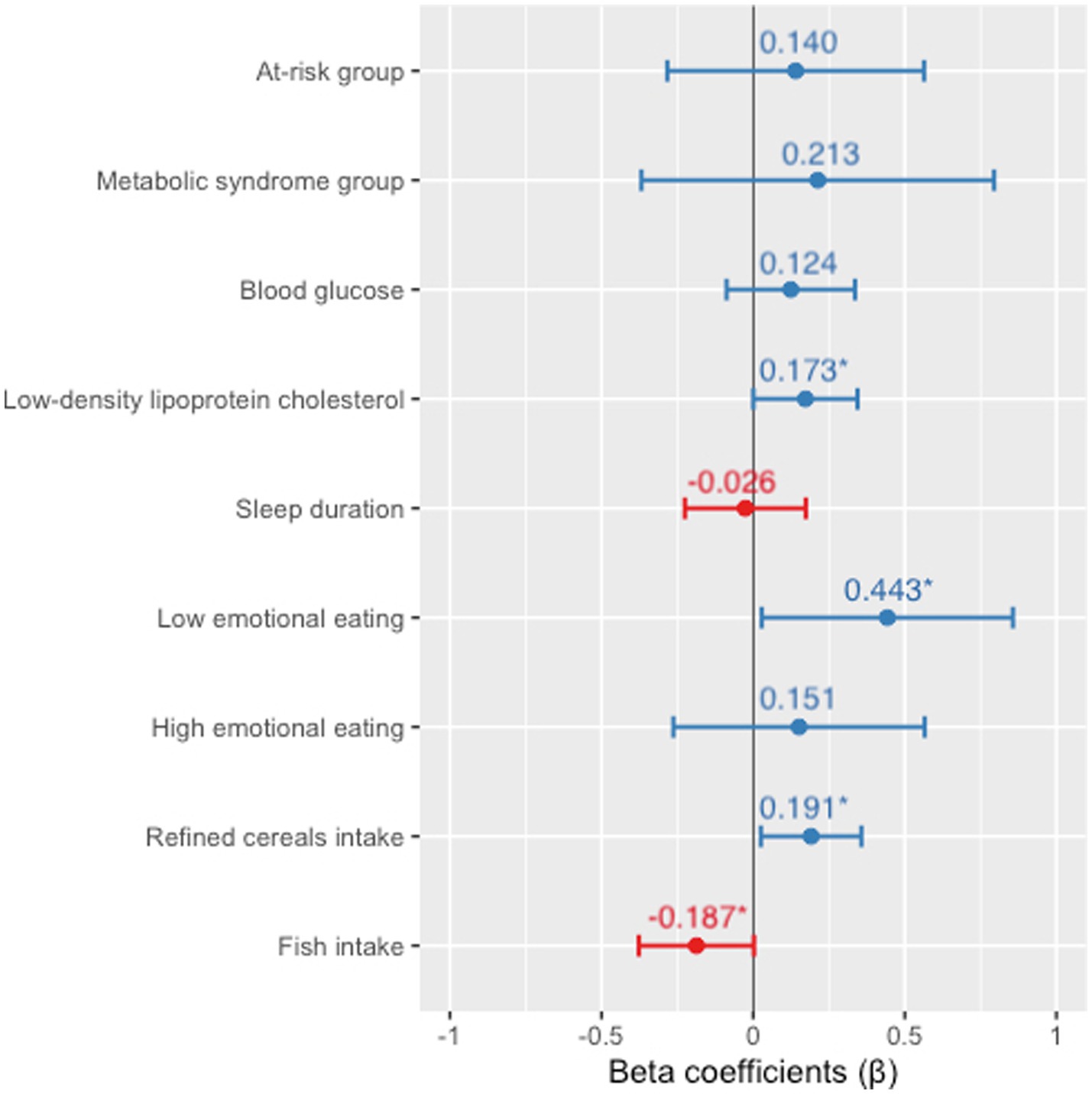
Figure 2. Multiple linear regression model for 8-isoprostane associations. Adjusted for sex and energy intake. Numerical variables are expressed as z-scores. *Statistically significant difference (value of p ≤0.05).
4. Discussion
Three principal observations were made in the course of the present study. First, 8-isoprostane was associated with an unhealthy metabolic status in the adolescent cohort, being positively related with adiposity (high z-scores for BMI, WHtR, and WC, and high body fat percentage), LDL-c, and MS. Second, an association between EE and 8-isoprostane was found, which to our knowledge has not been previously reported in adolescents. Finally, our results suggest that diet can significantly influence 8-isoprostane levels, which were positively associated with refined cereal intake and negatively associated with fish intake.
Consistent with our results, higher 8-isoprostane levels have been observed in obese children and adolescents compared to those with normal weight (35–37). This biomarker has also been positively correlated with measures of fatness, as well as WC, WHtR, and body fat (38–40). The association we found between oxidative stress, manifested by increased levels of 8-isoprostane, and MS in an adolescent population is also supported by previous studies in children (38, 41, 42) and adolescents (35), which report higher levels of 8-isoprostane in those with symptoms of MS. Moreover, overweight children with MS showed higher 8-isoprostane levels than overweight children without metabolic risk factors (42); it is possible that risk factors such as hyperglycemia, hypertriglyceridemia, presents in MS contribute to the presence of oxidative stress (10). Adolescents with a high BMI were observed to have higher levels of 8-isoprostane if they were insulin-resistant as opposed to insulin-sensitive, but no difference was observed with a low BMI and resistance/sensitivity to insulin (37), suggesting that both, adiposity and risk factors, could conduce to oxidative stress (10, 43). Therefore, it is possible that oxidative stress worsens with obesity, especially when coupled with MS risk factors. In the present study, consistent with the symptoms of MS, LDL-c was positively associated with 8-isoprostane; similar results have been reported in children with excess weight (38), children with diabetes mellitus type 1 (44), and adolescents with insulin resistance (45).
Although a relationship between high EE and MS has been described in adults (4, 46, 47), it remains poorly researched in adolescents. The most closely related study was carried out with adolescents diagnosed with type 1 diabetes, in whom higher EE values were associated with higher levels of HbA1, total cholesterol and LDL-c, which are risk factor of MS (48). On the other hand, an association between EE and obesity, which is the principal characteristic of MS, has been observed in adults (49–55), but has been scarcely studied in adolescents (56).
Since sleeping and physical activity are very important factors in the health of adolescents, we evaluated these variables and consistently with others results, we observed an association between 8-isoprostane and sleep duration (57) and between MS and sleep duration (58), not with physical activity. However, in the multiple linear regression analysis, physical activity or sleep duration were not significant factors in 8-isoprostane level.
As reported in the aforementioned studies, EE is linked with obesity. The association between obesity and 8-isoprostane could therefore explain the relationship found in the present study between EE and 8-isoprostane. The EE questionnaire evaluates the individual’s response to food consumption, focusing on the emotions of anxiety, loneliness, and depression. Previous research has shown a positive association between 8-isoprostane and anxiety as well as depression (59–63), which is in agreement with the results obtained here. A possible explanation of these relationships could be the effects of oxidative stress on the nervous system. The brain has a high rate of oxygen consumption and is rich in lipids, which contributes to the susceptibility of its cells to oxidative stress (64). The resulting inflammatory processes (65) alter the function of serotonin and dopamine, leading to symptoms of anxiety and depression (66–68), both of which are components of the items in the EE assessment (69, 70). Consequently, the directionality of the relationship between oxidative stress and EE is not yet clear. The scope of the present study is limited to demonstrating that an association exists between 8-isoprostane and EE; future work could shed more light on this relationship.
Diet is reported to modulate oxidative stress (15). Accordingly, a research work found a reduction in urinary isoprostanes and other oxidative stress biomarkers in MS patients who consumed a Mediterranean diet (71). In the present study, analysis of the eating habits of the adolescent participants revealed a positive association between 8-isoprostane and refined cereal intake. The quality of ingested carbohydrates is known to affect metabolic risk factors (72–75), which is consistent with our findings. A cross-over study in adults reported significantly higher levels of 8-isoprostane in consumers of a refined wheat diet compared to a wheat aleurone diet (76). However, other studies did not find different levels of 8-isoprostane between the consumers of ground flaxseed or wheat bran (77), whole-grain or refined-grain products (78), and whole or refined grain foods (79).
The inverse association between the oxidative stress biomarker 8-isoprostane and fish intake found in the present study is in accordance with prior reports of an inverse association between oxidative stress and fish intake (71, 80, 81) or supplementation with fish oil (80, 82, 83) or eicosapentaenoic acid or docosahexaenoic acid (81, 84, 85). Conversely, other studies have failed to find any significant associations between oxidative stress and the consumption of fish, including oil supplements (86–88). On the other hand, review articles show an inverse association between fish consumption and prevalence of MS (89) and heart failure (90, 91), which is supported by our results.
A strength of this study is that, to the best of our knowledge, a significant association between 8-isoprostane and EE, refined cereal intake, and fish intake has not been previously demonstrated in adolescents. Additionally, the study takes a holistic approach in which anthropometric and biochemical factors, emotional management, and eating habits are analyzed. Other strong points include the multicenter design and the use of a standardized protocol, which reduces information bias. The limitations of the study include the small size and cross-sectional design of the study population. Also, sometimes the participants did not wear the accelerometer while practicing water activities or a sport requiring its removal (e.g., judo, basketball). Finally, blood samples were not obtained no to use invasive methods due to the age of the cohort, so we were unable to analyze 8-isoprostane in plasma, inflammatory or neurotransmitter biomarkers, which would have provided greater insight into oxidative stress. The results of the present study may contribute to the development of educational programs focused on the establishment of healthier lifestyles in early life stages. The findings also indicate that more research is needed to understand the interaction between food choices, emotion management, and oxidative stress status in adolescents with good or poor metabolic health.
In conclusion, a significant positive association between 8-isoprostane and EE, refined cereal intake, indicators of adiposity (BMI z-score, WHtR z-score, body fat percentage), and MS, and a negative association between 8-isoprostane and fish intake in adolescents has been found. This shows that dietary patterns such as those exhibited in the Mediterranean diet could help to prevent cardiometabolic diseases.
Data availability statement
The datasets presented in this article are not readily available because there are restrictions on the availability of the data for the SI! Program study, due to signed consent agreements around data sharing, which only allow access to external researchers for studies following project purposes. Requests to access the datasets should be directed to Steering Committee gsantos@fundacionshe.org, rodrigo.fernandez@cnic.es, juanmiguel.fernandez@cnic.es, lamuela@ub.edu.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Instituto de Salud Carlos III in Madrid (CEI PI 35_2016), the Fundació Unió Catalana d’Hospitals (CEI 16/41), and the University of Barcelona (IRB00003099). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
RL-R, GS-B, JF-A, and RF-J designed the study, project administration, and funding acquisition. SR-G, EL-S, PB, AC-G, MdM, JF-A, and AT-R supervised the study implementation and data collection. SR-G performed statistical analysis. SR-G, AT-R, and RL-R contributed to writing–original draft manuscript. SR-G, RL-R, AT-R, EL-S, JM, PB, AC-G, JF-A, RF-J, JM-G, AR-L, and RE contributed to writing–interpreted the study results, review, and editing. All authors contributed to the article and approved the submitted version.
Funding
The SI! Program for Secondary Schools trial was supported by the SHE Foundation, the la Caixa Foundation (LCF/PR/CE16/10700001), the Fundació la Marató de TV3 (grant number 369/C/2016). Support was also provided by the Ministerio de Ciencia, Innovación y Universidades (PID2020-114022RB-I00), CIBEROBN from the Instituto de Salud Carlos III (ISCIII) from the Ministerio de Ciencia, Innovación y Universidades (AEI/FEDER, UE), and Generalitat de Catalunya. The CNIC is supported by the ISCIII, the Ministerio de Ciencia e Innovación (MCIN) and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033), and INSA-UB a María de Maeztu Center of Excellence (grant CEX2021-001234-M funded by MICIN/AEI/FEDER, UE). RF-J is recipient of grant PI22/01560 funded by the ISCIII and co-funded by the European Union. GS-B was the recipient of grant LCF/PR/MS19/12220001 funded by la Caixa Foundation (ID 100010434). AT-R is a Serra Húnter fellow. EL-S was a FI-SDUR (EMC/503/2021) fellowship from the Generalitat de Catalunya. JM-G is a recipient of grant FPU21/04891 (Ayudas para la formación de profesorado universitario, FPU-2021) from the Ministerio de Educación, Cultura y Deporte.
Acknowledgments
The authors especially thank all the volunteers and their families, teachers, and schools for their contribution to the SI! Program for Secondary Schools. The authors thank the SHE Foundation (intellectual owner of the SI! Program) and its partners Isabel Carvajal, Domènec Haro, Xavier Orrit, Carla Rodríguez, Vanessa Carral, Rosa Casas, Carolina E. Storniolo, for their contribution to the study design, coordination, development, and all personnel who performed measurements in adolescents at participating schools.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1216445/full#supplementary-material
Abbreviations
BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; EE, emotional eating; HDL-c, high density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; MS, metabolic syndrome; SBP, systolic blood pressure; WC, waist circumference; WHtR, waist-to-height ratio.
References
1. O’Neil, A, Quirk, SE, Housden, S, Brennan, SL, Williams, LJ, Pasco, JA, et al. Relationship between diet and mental health in children and adolescents: a systematic review. Am J Public Health. (2014) 104:e31–42. doi: 10.2105/AJPH.2014.302110
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sawyer, SM, Afifi, RA, Bearinger, LH, Blakemore, SJ, Dick, B, Ezeh, AC, et al. Adolescence: a foundation for future health. Lancet. (2012) 379:1630–40. doi: 10.1016/S0140-6736(12)60072-5
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Kang, NR, and Kwack, YS. An update on mental health problems and cognitive behavioral therapy in pediatric obesity. Pediatr Gastroenterol Hepatol Nutr. (2020) 23:15. doi: 10.5223/pghn.2020.23.1.15
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Lopez-Cepero, A, Frisard, CF, Lemon, SC, and Rosal, MC. Association of dysfunctional eating patterns and metabolic risk factors for cardiovascular disease among Latinos. J Acad Nutr Diet. (2018) 118:849–56. doi: 10.1016/j.jand.2017.06.007
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Liu, T, Zhong, S, Liao, X, Chen, J, He, T, Lai, S, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. (2015) 10:e0138904. doi: 10.1371/journal.pone.0138904
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Khalid, S, Williams, CM, and Reynolds, SA. Is there an association between diet and depression in children and adolescents? A systematic review. Br J Nutr. (2016) 116:2097–108. doi: 10.1017/S0007114516004359
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Noubiap, JJ, Nansseu, JR, Lontchi-Yimagou, E, Nkeck, JR, Nyaga, UF, Ngouo, AT, et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. Lancet Child Adolesc Health. (2022) 6:158–70. doi: 10.1016/S2352-4642(21)00374-6
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Zimmet, P, Alberti, KGM, Kaufman, F, Tajima, N, Silink, M, Arslanian, S, et al. The metabolic syndrome in children and adolescents–an IDF consensus report. Pediatr Diabetes. (2007) 8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Raut, SK, and Khullar, M. Oxidative stress in metabolic diseases: current scenario and therapeutic relevance. Mol Cell Biochem. (2023) 478:185–96. doi: 10.1007/s11010-022-04496-z
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Shaito, A, Aramouni, K, Assaf, R, Parenti, A, Orekhov, A, El Yazbi, A, et al. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci. (2022) 27:0105. doi: 10.31083/j.fbl2703105
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Costabile, G, Della Pepa, G, Bozzetto, L, Annuzzi, G, Vetrani, C, Giacco, R, et al. Urine 8-isoprostane in relation to adiposity and insulin resistance in individuals at high cardiometabolic risk. Metab Syndr Relat Disord. (2015) 13:187–91. doi: 10.1089/met.2014.0119
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Ercan, H, Kiyici, A, Marakoglu, K, and Oncel, M. 8-Isoprostane and coenzyme Q10 levels in patients with metabolic syndrome. Metab Syndr Relat Disord. (2016) 14:318–21. doi: 10.1089/met.2016.0011
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Czerska, M, Zieliński, M, and Gromadzińska, J. Isoprostanes–a novel major group of oxidative stress markers. Int J Occup Med Environ Health. (2016) 29:179–90. doi: 10.13075/ijomeh.1896.00596
CrossRef Full Text | Google Scholar
16. Selvaraju, V, Ayine, P, Fadamiro, M, Babu, JR, Brown, M, and Geetha, T. Urinary biomarkers of inflammation and oxidative stress are elevated in obese children and correlate with a marker of endothelial dysfunction. Oxidative Med Cell Longev. (2019) 2019:1–10. doi: 10.1155/2019/9604740
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Van’t Erve, TJ, Kadiiska, MB, London, SJ, and Mason, RP. Classifying oxidative stress by F2-isoprostane levels across human diseases: a meta-analysis. Redox Biol. (2017) 12:582–99. doi: 10.1016/j.redox.2017.03.024
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Fernandez-Jimenez, R, Santos-Beneit, G, Tresserra-Rimbau, A, Bodega, P, de Miguel, M, de Cos-Gandoy, A, et al. Rationale and design of the school-based SI! Program to face obesity and promote health among Spanish adolescents: a cluster-randomized controlled trial. Am Heart J. (2019) 215:27–40. doi: 10.1016/j.ahj.2019.03.014
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Cole, TJ, and Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity: extended international BMI cut-offs. Pediatr Obes. (2012) 7:284–94. doi: 10.1111/j.2047-6310.2012.00064.x
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Sharma, AK, Metzger, DL, Daymont, C, Hadjiyannakis, S, and Rodd, CJ. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. (2015) 78:723–9. doi: 10.1038/pr.2015.160
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Whitehead, SJ, Ford, C, and Gama, R. A combined laboratory and field evaluation of the Cholestech LDX and CardioChek PA point-of-care testing lipid and glucose analysers. Ann Clin Biochem. (2014) 51:54–67. doi: 10.1177/0004563213482890
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Medina-Remón, A, Barrionuevo-González, A, Zamora-Ros, R, Andres-Lacueva, C, Estruch, R, Martínez-González, MÁ, et al. Rapid Folin–Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal Chim Acta. (2009) 634:54–60. doi: 10.1016/j.aca.2008.12.012
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Flynn, JT, Kaelber, DC, Baker-Smith, CM, Blowey, D, Carroll, AE, Daniels, SR, et al. Clinical practice guideline for screening and Management of High Blood Pressure in children and adolescents. JAMA Pediatr. (2018) 172:1087. doi: 10.1001/jamapediatrics.2018.2882
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Rosner, B, Cook, N, Portman, R, Daniels, S, and Falkner, B. Determination of blood pressure percentiles in Normal-weight children: some methodological issues. Am J Epidemiol. (2008) 167:653–66. doi: 10.1093/aje/kwm348
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Chandler, JL, Brazendale, K, Beets, MW, and Mealing, BA. Classification of physical activity intensities using a wrist-worn accelerometer in 8-12-year-old children: wrist-worn accelerometry in children. Pediatr Obes. (2016) 11:120–7. doi: 10.1111/ijpo.12033
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Sadeh, A, Sharkey, M, and Carskadon, MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. (1994) 17:201–7. doi: 10.1093/sleep/17.3.201
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Rajmil, L, Serra-Sutton, V, Alonso, J, Herdman, M, Riley, A, and Starfield, B. Validity of the Spanish version of the child health and illness profile-adolescent edition (CHIP-AE). Med Care. (2003) 41:1153–63. doi: 10.1097/01.MLR.0000088460.42155.65
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Jáuregui-Lobera, I, García-Cruz, P, Carbonero-Carreño, R, Magallares, A, and Ruiz-Prieto, I. Psychometric properties of Spanish version of the three-factor eating questionnaire-R18 (Tfeq-Sp) and its relationship with some eating-and body image-related variables. Nutrients. (2014) 6:5619–35. doi: 10.3390/nu6125619
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Ahonen, EQ, Nebot, M, and Giménez, E. Negative mood states and related factors in a sample of adolescent secondary-school students in Barcelona (Spain). Gac Sanit. (2007) 21:43–52. doi: 10.1157/13099120
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Fernández-Ballart, JD, Piñol, JL, Zazpe, I, Corella, D, Carrasco, P, Toledo, E, et al. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr. (2010) 103:1808–16. doi: 10.1017/S0007114509993837
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Juton, C, Castro-Barquero, S, Casas, R, Freitas, T, Ruiz-León, AM, Crovetto, F, et al. Reliability and concurrent and construct validity of a food frequency questionnaire for pregnant women at high risk to develop fetal growth restriction. Nutrients. (2021) 13:1629. doi: 10.3390/nu13051629
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Farran, A, Ral, PC, and Zamora, R. Tablas de composición de alimentos del CESNID. New York: McGraw-Hill Interamericana (2003).
Google Scholar
34. Moreiras, O, Carbajal, Á, Cabrera, L, and Cuadrado, C. Tablas de composición de alimentos In: Guía de Prácticas. 19th ed. Madrid, Spain: Pirámide (2018)
Google Scholar
35. Karamouzis, I, Pervanidou, P, Berardelli, R, Iliadis, S, Papassotiriou, I, Karamouzis, M, et al. Enhanced oxidative stress and platelet activation combined with reduced antioxidant capacity in obese prepubertal and adolescent girls with full or partial metabolic syndrome. Horm Metab Res. (2011) 43:607–13. doi: 10.1055/s-0031-1284355
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Wu, HC, Brennan, LA, Goldberg, M, Chung, WK, Wei, Y, Santella, RM, et al. Influence of pubertal development on urinary oxidative stress biomarkers in adolescent girls in the New York LEGACY cohort. Free Radic Res. (2020) 54:431–41. doi: 10.1080/10715762.2020.1798001
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Sinaiko, AR, Steinberger, J, Moran, A, Prineas, RJ, Vessby, B, Basu, S, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. (2005) 111:1985–91. doi: 10.1161/01.CIR.0000161837.23846.57
PubMed Abstract | CrossRef Full Text | Google Scholar
38. Dennis, BA, Ergul, A, Gower, BA, Allison, JD, and Davis, CL. Oxidative stress and cardiovascular risk in overweight children in an exercise intervention program. Child Obes. (2013) 9:15–21. doi: 10.1089/chi.2011.0092
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Ostrow, V, Wu, S, Aguilar, A, Bonner, R Jr, Suarez, E, and De Luca, F. Association between oxidative stress and masked hypertension in a multi-ethnic population of obese children and adolescents. J Pediatr. (2011) 158:628–633.e1. doi: 10.1016/j.jpeds.2010.09.081
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Polidori, N, Giannini, C, Salvatore, R, Pelliccia, P, Parisi, A, Chiarelli, F, et al. Role of urinary NGAL and KIM-1 as biomarkers of early kidney injury in obese prepubertal children. J Pediatr Endocrinol Metab. (2020) 33:1183–9. doi: 10.1515/jpem-2020-0138
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Araki, S, Dobashi, K, Yamamoto, Y, Asayama, K, and Kusuhara, K. Increased plasma isoprostane is associated with visceral fat, high molecular weight adiponectin, and metabolic complications in obese children. Eur J Pediatr. (2010) 169:965–70. doi: 10.1007/s00431-010-1157-z
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Kelly, AS, Steinberger, J, Kaiser, DR, Olson, TP, Bank, AJ, and Dengel, DR. Oxidative stress and adverse adipokine profile characterize the metabolic syndrome in children. J Cardiometab Syndr. (2006) 1:248–52. doi: 10.1111/j.1559-4564.2006.05758.x
PubMed Abstract | CrossRef Full Text | Google Scholar
43. Yu, J, Qiu, J, Zhang, Z, Cui, X, Guo, W, Sheng, M, et al. Redox biology in adipose tissue physiology and obesity. Adv Biol. (2023) 2023:2200234. doi: 10.1002/adbi.202200234
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Wu, D, Xiu, GC, Meng, X, and Yang, QL. Correlation between blood glucose fluctuations and activation of oxidative stress in type 1 diabetic children during the acute metabolic disturbance period. Chin Med J. (2013) 126:4019–22. doi: 10.3760/cma.j.issn.0366-6999.20131841
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Acosta-García, E, Carías, D, Páez, M, Naddaf, G, and Domínguez, Z. Peroxidación lipídica en adolescentes púberes. Rev Salud Pública. (2018) 20:623–8. doi: 10.15446/rsap.v20n5.63476
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Akıllıoğlu, T, Bas, M, and Köse, G. Restrained, emotional eating and depression can be a risk factor for metabolic syndrome. Nutr Hosp. (2022) 39:1264–71. doi: 10.20960/nh.03947
PubMed Abstract | CrossRef Full Text | Google Scholar
48. Ripoli, C, Ricciardi, MR, Zuncheddu, E, Angelo, MR, Pinna, AP, and Ripoli, D. Emotional eating and disordered eating behaviors in children and adolescents with type 1 diabetes. Sci Rep. (2022) 12:21854. doi: 10.1038/s41598-022-26271-2
PubMed Abstract | CrossRef Full Text | Google Scholar
49. du, C, Adjepong, M, Zan, MCH, Cho, MJ, Fenton, JI, Hsiao, PY, et al. Gender differences in the relationships between perceived stress, eating behaviors, sleep, dietary risk, and body mass index. Nutrients. (2022) 14:1045. doi: 10.3390/nu14051045
PubMed Abstract | CrossRef Full Text | Google Scholar
50. Jones, J, Kauffman, BY, Rosenfield, D, Smits, JA, and Zvolensky, MJ. Emotion dysregulation and body mass index: the explanatory role of emotional eating among adult smokers. Eat Behav. (2019) 33:97–101. doi: 10.1016/j.eatbeh.2019.05.003
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Kornacka, M, Czepczor-Bernat, K, Napieralski, P, and Brytek-Matera, A. Rumination, mood, and maladaptive eating behaviors in overweight and healthy populations. Eat Weight Disord. (2021) 26:273–85. doi: 10.1007/s40519-020-00857-z
PubMed Abstract | CrossRef Full Text | Google Scholar
52. Martins, BG, da Silva, WR, Maroco, J, and Campos, JADB. Psychometric characteristics of the three-factor eating Questionnaire-18 and eating behavior in undergraduate students. Eat Weight Disord. (2021) 26:525–36. doi: 10.1007/s40519-020-00885-9
PubMed Abstract | CrossRef Full Text | Google Scholar
53. Pacheco, LS, Blanco, E, Burrows, R, Correa-Burrows, P, Santos, JL, and Gahagan, S. Eating behavior and body composition in Chilean young adults. Appetite. (2021) 156:104857. doi: 10.1016/j.appet.2020.104857
PubMed Abstract | CrossRef Full Text | Google Scholar
55. Vega, JA, Salazar, G, Hodgson, MI, Cataldo, LR, Valladares, M, Obregón, AM, et al. Melanocortin-4 receptor gene variation is associated with eating behavior in Chilean adults. Ann Nutr Metab. (2016) 68:35–41. doi: 10.1159/000439092
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Swartz, MC, Basen-Engquist, KM, Markham, C, Lyons, EJ, Cox, M, Chandra, J, et al. Psychometric analysis of the three-factor eating questionnaire-R18V2 in adolescent and young adult-aged central nervous system tumor survivors. J Adolesc Young Adult Oncol. (2016) 5:278–85. doi: 10.1089/jayao.2015.0058
PubMed Abstract | CrossRef Full Text | Google Scholar
57. Nagata, C, Tamura, T, Wada, K, Konishi, K, Goto, Y, Nagao, Y, et al. Sleep duration, nightshift work, and the timing of meals and urinary levels of 8-isoprostane and 6-sulfatoxymelatonin in Japanese women. Chronobiol Int. (2017) 34:1187–96. doi: 10.1080/07420528.2017.1355313
PubMed Abstract | CrossRef Full Text | Google Scholar
58. Hua, J, Jiang, H, Wang, H, and Fang, Q. Sleep duration and the risk of metabolic syndrome in adults: a systematic review and meta-analysis. Front Neurol. (2021) 12:635564. doi: 10.3389/fneur.2021.635564
PubMed Abstract | CrossRef Full Text | Google Scholar
59. Diniz, BS, Mendes-Silva, AP, Silva, LB, Bertola, L, Vieira, MC, Ferreira, JD, et al. Oxidative stress markers imbalance in late-life depression. J Psychiatr Res. (2018) 102:29–33. doi: 10.1016/j.jpsychires.2018.02.023
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Lopresti, AL, Maker, GL, Hood, SD, and Drummond, PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Prog Neuro-Psychopharmacol Biol Psychiatry. (2014) 48:102–11. doi: 10.1016/j.pnpbp.2013.09.017
PubMed Abstract | CrossRef Full Text | Google Scholar
61. Savage, K, Kingshott, D, Gubko, A, Thee, AW, Burjawi, T, Croft, K, et al. The relationship between oxidative stress and anxiety in a healthy older population. Exp Aging Res. (2021) 47:322–46. doi: 10.1080/0361073X.2021.1883966
PubMed Abstract | CrossRef Full Text | Google Scholar
62. Savage, K, Gogarty, L, Lea, A, Deleuil, S, Nolidin, K, Croft, K, et al. The relationship between F2-Isoprostanes plasma levels and depression symptoms in healthy older adults. Antioxidants. (2022) 11:822. doi: 10.3390/antiox11050822
PubMed Abstract | CrossRef Full Text | Google Scholar
63. Steenkamp, LR, Hough, CM, Reus, VI, Jain, FA, Epel, ES, James, SJ, et al. Severity of anxiety–but not depression–is associated with oxidative stress in major depressive disorder. J Affect Disord. (2017) 219:193–200. doi: 10.1016/j.jad.2017.04.042
PubMed Abstract | CrossRef Full Text | Google Scholar
65. Das Fedoce, AG, Ferreira, F, Bota, RG, Bonet-Costa, V, Sun, PY, and Davies, KJ. The role of oxidative stress in anxiety disorder: cause or consequence? Free Radic Res. (2018) 52:737–50. doi: 10.1080/10715762.2018.1475733
PubMed Abstract | CrossRef Full Text | Google Scholar
66. Leonard, B, and Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. (2012) 36:764–85. doi: 10.1016/j.neubiorev.2011.12.005
PubMed Abstract | CrossRef Full Text | Google Scholar
67. Robson, MJ, Quinlan, MA, and Blakely, RD. Immune system activation and depression: roles of serotonin in the central nervous system and periphery. ACS Chem Neurosci. (2017) 8:932–42. doi: 10.1021/acschemneuro.6b00412
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Ohrt, TK, Perez, M, Liew, J, Hernández, JC, and Yu, KY. The influence of temperament on stress-induced emotional eating in children. Obes Sci Pract. (2020) 6:524–34. doi: 10.1002/osp4.439
PubMed Abstract | CrossRef Full Text | Google Scholar
70. Vainik, U, García-García, I, and Dagher, A. Uncontrolled eating: a unifying heritable trait linked with obesity, overeating, personality and the brain. Eur J Neurosci. (2019) 50:2430–45. doi: 10.1111/ejn.14352
PubMed Abstract | CrossRef Full Text | Google Scholar
71. Mitjavila, MT, Fandos, M, Salas-Salvadó, J, Covas, MI, Borrego, S, Estruch, R, et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin Nutr. (2013) 32:172–8. doi: 10.1016/j.clnu.2012.08.002
PubMed Abstract | CrossRef Full Text | Google Scholar
72. Giacco, R, Costabile, G, Della Pepa, G, Anniballi, G, Griffo, E, Mangione, A, et al. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr Metab Cardiovasc Dis. (2014) 24:837–44. doi: 10.1016/j.numecd.2014.01.007
PubMed Abstract | CrossRef Full Text | Google Scholar
73. Marshall, S, Petocz, P, Duve, E, Abbott, K, Cassettari, T, Blumfield, M, et al. The effect of replacing refined grains with whole grains on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials with GRADE clinical recommendation. J Acad Nutr Diet. (2020) 120:1859–1883.e31. doi: 10.1016/j.jand.2020.06.021
PubMed Abstract | CrossRef Full Text | Google Scholar
74. Martínez-González, MA, Fernandez-Lazaro, CI, Toledo, E, Díaz-López, A, Corella, D, Goday, A, et al. Carbohydrate quality changes and concurrent changes in cardiovascular risk factors: a longitudinal analysis in the PREDIMED-plus randomized trial. Am J Clin Nutr. (2020) 111:291–306. doi: 10.1093/ajcn/nqz298
PubMed Abstract | CrossRef Full Text | Google Scholar
75. Soujanya, KV, and Jayadeep, AP. Obesity-associated biochemical markers of inflammation and the role of grain phytochemicals. J Food Biochem. (2022) 46:e14257. doi: 10.1111/jfbc.14257
PubMed Abstract | CrossRef Full Text | Google Scholar
76. Costabile, G, Vitale, M, Della Pepa, G, Cipriano, P, Vetrani, C, Testa, R, et al. A wheat aleurone-rich diet improves oxidative stress but does not influence glucose metabolism in overweight/obese individuals: results from a randomized controlled trial. Nutr Metab Cardiovasc Dis. (2022) 32:715–26. doi: 10.1016/j.numecd.2021.12.016
PubMed Abstract | CrossRef Full Text | Google Scholar
77. Bloedon, LT, Balikai, S, Chittams, J, Cunnane, SC, Berlin, JA, Rader, DJ, et al. Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr. (2008) 27:65–74. doi: 10.1080/07315724.2008.10719676
PubMed Abstract | CrossRef Full Text | Google Scholar
78. Andersson, A, Tengblad, S, Karlström, B, Kamal-Eldin, A, Landberg, R, Basu, S, et al. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr. (2007) 137:1401–7. doi: 10.1093/jn/137.6.1401
CrossRef Full Text | Google Scholar
79. Enright, L, and Slavin, J. No effect of 14 day consumption of whole grain diet compared to refined grain diet on antioxidant measures in healthy, young subjects: a pilot study. Nutr J. (2010) 9:1–8. doi: 10.1186/1475-2891-9-12
PubMed Abstract | CrossRef Full Text | Google Scholar
80. Hansson, P, Barregård, L, Halltorp, M, Sibthorpe, S, Svelander, C, Sandberg, AS, et al. Habitual high intake of fatty fish is related to lower levels of F2-isoprostane in healthy women. Nutrition. (2015) 31:847–52. doi: 10.1016/j.nut.2014.12.015
PubMed Abstract | CrossRef Full Text | Google Scholar
81. Mori, TA, Dunstan, DW, Burke, V, Croft, KD, Rivera, JH, Beilin, LJ, et al. Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non—insulin-dependent diabetic patients. Metabolism. (1999) 48:1402–8. doi: 10.1016/S0026-0495(99)90150-6
PubMed Abstract | CrossRef Full Text | Google Scholar
82. Deshpande, G, Simmer, K, Deshmukh, M, Mori, TA, Croft, KD, and Kristensen, J. Fish oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr. (2014) 58:177–82. doi: 10.1097/MPG.0000000000000174
PubMed Abstract | CrossRef Full Text | Google Scholar
83. Pipingas, A, Sinclair, A, Croft, KD, Januszewski, AS, Jenkins, AJ, Mori, TA, et al. Fish oil and multivitamin supplementation reduces oxidative stress but not inflammation in healthy older adults: a randomised controlled trial. J Funct Foods. (2015) 19:949–57. doi: 10.1016/j.jff.2014.10.028
CrossRef Full Text | Google Scholar
84. Guillot, N, Caillet, E, Laville, M, Calzada, C, Lagarde, M, and Véricel, E. Increasing intakes of the long-chain ω-3 docosahexaenoic acid: effects on platelet functions and redox status in healthy men. FASEB J. (2009) 23:2909–16. doi: 10.1096/fj.09-133421
PubMed Abstract | CrossRef Full Text | Google Scholar
85. Purcell, R, Latham, SH, Botham, KM, Hall, WL, and Wheeler-Jones, CP. High-fat meals rich in EPA plus DHA compared with DHA only have differential effects on postprandial lipemia and plasma 8-isoprostane F2α concentrations relative to a control high–oleic acid meal: a randomized controlled trial. Am J Clin Nutr. (2014) 100:1019–28. doi: 10.3945/ajcn.114.091223
PubMed Abstract | CrossRef Full Text | Google Scholar
86. Conway, MC, McSorley, EM, Mulhern, MS, Spence, T, Wijngaarden, E, Watson, GE, et al. The influence of fish consumption on serum n-3 polyunsaturated fatty acid (PUFA) concentrations in women of childbearing age: a randomised controlled trial (the iFish study). Eur J Nutr. (2021) 60:1415–27. doi: 10.1007/s00394-020-02326-w
PubMed Abstract | CrossRef Full Text | Google Scholar
87. Erkkilä, AT, Lee, JC, Lankinen, M, Manninen, S, Leung, HH, Oger, C, et al. Camelina sativa oil, fatty fish, and lean fish do not markedly affect urinary prostanoids in subjects with impaired glucose metabolism. Lipids. (2019) 54:453–64. doi: 10.1002/lipd.12176
PubMed Abstract | CrossRef Full Text | Google Scholar
88. Wu, W, Lu, S, Wang, T, Jou, H, and Wang, T. Effects of docosahexaenoic acid supplementation on blood lipids, estrogen metabolism, and in vivo oxidative stress in postmenopausal vegetarian women. Eur J Clin Nutr. (2006) 60:386–92. doi: 10.1038/sj.ejcn.1602328
PubMed Abstract | CrossRef Full Text | Google Scholar
89. Tørris, C, Småstuen, MC, and Molin, M. Nutrients in fish and possible associations with cardiovascular disease risk factors in metabolic syndrome. Nutrients. (2018) 10:952. doi: 10.3390/nu10070952
PubMed Abstract | CrossRef Full Text | Google Scholar
90. He, K, Song, Y, Daviglus, ML, Liu, K, van Horn, L, Dyer, AR, et al. Fish consumption and incidence of stroke. Stroke. (2004) 35:1538–42. doi: 10.1161/01.STR.0000130856.31468.47
PubMed Abstract | CrossRef Full Text | Google Scholar
91. Kerley, CP
. Dietary patterns and components to prevent and treat heart failure: a comprehensive review of human studies. Nutr Res Rev. (2019) 32:1–27. doi: 10.1017/S0954422418000148
PubMed Abstract | CrossRef Full Text | Google Scholar