This research complies with and was approved by Stanford’s Institutional Review Board (IRB-46563). The study protocol for this clinical trial (trial registration no. NCT03868670) is publicly available as a supplement to this manuscript. Data presented in this manuscript can be requested within reason by contacting the corresponding author (see Data availability for additional details). As previously described, the clinical trial is a multi-stage early feasibility study with staggered enrollment (Fig. 1a, see protocol paper for more details17). All participant information, magnetic resonance imaging (MRI), computed tomography (CT), LFP and behavior data were collected under a Stanford University IRB-approved protocol (IRB no. 46563, NCT03868670).
Prestudy procedures
Subjects
Two adult women with BED and treatment-refractory, severe (grade III) obesity, despite bariatric surgery, were recruited for the present study. The first participant (subject 1), a 45-year-old white woman with a BMI of 46 kg m−2, underwent roux-en-Y gastric bypass (RYGB) in 2005 and experienced an initial weight loss of 52.16 kg. However, the participant gradually regained the weight. At the time of enrollment, she was back to her pre-RYGB weight and met DSM-V criteria1 for BED, reporting at least five LOC eating bouts per week, with three LOC eating events per week as well. At the time of enrollment, she had co-morbities of neoplasm, lower back pain, kyphoscoliosis/scoliosis, hypertension, esophageal reflux, dyslipidemia, complicated migraine and anxiety. The second participant (subject 2), a 56-year-old white woman with a BMI of 47 kg m−2, underwent RYGB in 2005 and initially lost 68.95 kg. Subject 2 maintained this weight loss for 6 years; however, she regained the weight in 2009 while both caring for an ill family member and recovering from a car accident. At the time of study enrollment, she was within 9% of her pre-RYGB weight and she reported LOC eating four times a week and met criteria for BED as well. At the time of enrollment, she had a migraine co-morbidity. Both subjects reported severe cravings related to emotional and/or stress-related triggers that led to LOC eating. In addition to the RYGB, both subjects tried numerous other weight-loss strategies including exercise, dieting, support groups and medication. as was required by our enrollment criteria.
The study was approved by Stanford’s IRB (IRB-46563) (see Supplementary Information for participant characteristics) and informed consent was obtained from all subjects. Neither participant was compensated for their participation.
Study design
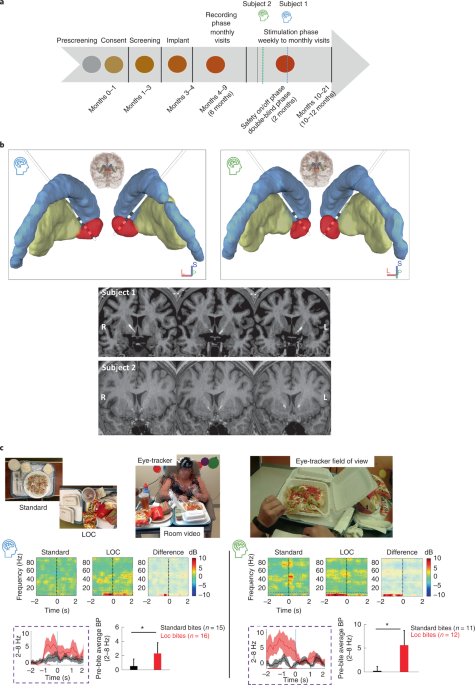
Designed with a staggered enrollment, each subject progressed through the study stages shown in Fig. 1a. Both subjects underwent stereotactic implantation of bilateral depth electrodes, each with four contacts. The two distal contacts of the DBS leads were positioned in the NAc, verified by both indirect and direct targeting strategies noted earlier, with the two more proximal contacts traversing the anterior limb of the internal capsule (Fig. 1b and Supplementary Table 2)22. The preoperative MRI anatomical images were co-registered to a post-surgical CT scan using advanced normalization tools (ANTs23) for electrode localization24. The electrode artifacts from the co-registered postoperative CT, together with the preoperative MRI and atlas-based regions of interest, were then loaded in DSI Studio for three-dimensional rendering (https://dsi-studio.labsolver.org), as presented in Fig. 1b.
Implantation → Recording phase (6 months) → Stimulation phase (12 months; see Fig. 1a).
Responsive DBS system
The closed-loop intracranial stimulation system used here for rDBS was a brain RNS System (NeuroPace, Inc.) currently approved by the US FDA for the treatment of adults with medically refractory focal-onset seizures. The system consists of two intracranial recording and stimulating electrodes connected to a neurostimulator implanted in the skull. The neurostimulator continuously monitors electrographic signals and detects abnormal electrophysiological activity to trigger brief bursts of electrical stimulation. The RNS System stores hourly counts of detected events as well as multiple 1- to 3-min snapshots of LFP activity, which are uploaded to a computer and server where they can be analyzed and reviewed. This system allows the opportunity to collect and examine electrographic activity of the NAc in a subject’s naturalistic environment during vulnerable periods of craving that precede LOC eating events.
Stereotactic targeting, device implantation and recording configuration
After screening by a multidisciplinary team, the subjects underwent surgical placement of the cranial neurostimulator attached to two depth leads (four contacts each, 3.5-mm electrode spacing) (NeuroPace, Inc.) targeting the NAc bilaterally (Fig. 1b). Direct targeting of the NAc using Fast Gray Matter Acquisition T1 Inversion Recovery (fGMATI) MRI was implemented. Due to the investigative nature of the present study, the neurostimulator (250-Hz sampling rate) was programmed with the high-pass filter set to 1 Hz and the low-pass filter set to 90 Hz, the widest range available on the device. From each hemisphere, the neurostimulator stores two channels of differential recordings each made up of one NAc electrode pair (contacts 1 and 2) and another electrode pair in the white matter of the anterior limb of the internal capsule (contacts 3 and 4) to make up a ventral NAc (contacts 1 and 3) and dorsal NAc (contacts 2 and 4) recording. Implantation was performed for subject 1 on 31 January 2020 and for subject 2 on 17 July 2020.
Recording phase
Immediately after implantation, the subjects entered a 6-month recording phase, during which naturalistic in-lab assessments and ambulatory real-world assessments were performed to identify an electrophysiological biomarker for rDBS in the consecutive stimulation phase. From each hemisphere, activity was recorded from the ventral and dorsal NAc (see Supplementary Information for details).
During the end of the recording phase of the study and after optimization of signal detection, both subjects underwent a day of monopolar survey testing of all the electrode contacts to screen for any acute stimulation effects. One week after this survey testing, each subject was randomized in a single-blind fashion to receive a week of active or sham rDBS unilaterally to assess initial tolerability and optimize stimulation parameters. One subject was randomized to active stimulation and the other sham stimulation. For subject 1, stimulation was administered at 3 mA unilateral for a week, off for a week, 5 mA unilateral for 4 weeks. The subject was unblinded after these 4 weeks and transitioned to open-label stimulation. For subject 2, stimulation was administered at 0.5 mA unilateral for a week, off for a week, 1 mA bilateral (split between two leads) for 4 weeks. The subject randomized to sham stimulation underwent a week of active stimulation testing before transitioning into the open-label phase. Stimulation was well tolerated throughout this protocol with no adverse effects.
Behavioral Assessments
Subjects underwent two assessments to evaluate NAc electrophysiology during: (1) anticipation (pre-consumption) of food during standard meals and LOC eating (that is, multi-item buffet assessment, in-lab naturalistic testing); and (2) states of hunger and craving (pre-consumption) (that is, ambulatory assessment, real-world testing). All behavioral assessments were collected during the recording phase (see Fig. 1a).
Multi-item buffet
Subjects participated in a modified multi-item buffet designed to provoke LOC eating in a more naturalistic setting (developed by W. Stuart Agras18). This buffet of preferred foods was intended to model the at-risk environment of LOC in a controlled setting where real-time brain recordings could be synchronized to video monitoring. For this task, subjects spent the day in this behavioral lab where they were served a breakfast and lunch (~1,000 kcal total). After a mood provocation protocol with an eating disorder psychiatrist (D.S.), the subjects were presented with a 5,000-kcal calorie buffet of preferred foods. The mood provocation was administered based on research showing that negative affective states, compared with neutral mood provocations, are more likely to trigger loss of control/disinhibited eating in patients who binge eat18. During the buffet, real-time video with eye tracking (Pupil Lab Pupil Core Eyetracker (https://pupil-labs.com/products/core) and room video) and NAc LFP activity were recorded to capture bites (see Fig. 1c, picture insert25). Subjects reported their level of LOC before, during and after the presentation of the foods. The buffet was stopped 15 min after the subject would verbalize that they were experiencing LOC per FDA safety guidelines.
Ambulatory assessment
Due to storage capacity limitations, the neurostimulator does not store continuous LFP activity; however, it does record multiple LFP snapshots of activity when an event is detected, based on time of day or when the subject triggers storage (by swiping a magnet over the device). To assess LOC in the real world, subjects were instructed to trigger LFP activity storage (180 s for subject 1, 90 s for subject 2) whenever they had a craving and were about to eat. The storage time was different for each subject based on understanding the subject’s LOC behavior (for example, subject 2’s LOC revolved around multiple snacking events so they would report LOC more frequently) and how many events we could store without losing data. The subjects noted the day and time of the events in a diary and also provided Likert ratings of LOC severity, craving and hunger during these times. In addition to swiping the magnet for cravings, subjects were asked to swipe the magnet when they felt relaxed/‘normal’ to timestamp control periods. Detections were also scheduled to be stored during a randomly selected time of the day during awake (awake detections) and sleep (sleep detections) hours. Both of these events are scheduled LFP recordings that are not user initiated and not tied to an LOC event. As such, low-frequency detections acquired during these scheduled events act as controls to LOC detections and user-initiated events. Our primary analyses were designed to compare NAc LFP activity recorded during reported LOC versus control period magnet swipes. For both subjects, LOC was identified early on to be frequently associated with events of craving in the absence of hunger. In fact, both subjects noted qualitative differences in craving severity with higher severity cravings (referred to as high craving) being associated with LOC-related binges historically. Thus, for our study purposes, the presence of high craving with no hunger (that is, subjects felt at risk of losing control even though they were not hungry) was defined as an at-risk moment, and is referred to as a ‘LOC eating event’. In contrast, events of hunger sensations even in the context of craving (potentially closer to ‘typical eating’ behavior in the presence of metabolic need) were not associated with reported LOC. Subject reporting of LOC using the magnet swipe feature is probably one of the first of its kind, and a very new method for assessing ambulatory, real-world conditions.
Stimulation phase
After the recording phase, both subjects underwent single-blinded stimulation survey testing in which they received brief bursts of electrical stimulation across all electrode contacts to screen for acute effects. This was followed by a single-blinded, staged, on–off stimulation safety testing period to assess for possible side effects of rDBS. Subjects then entered the 10- to 12-month open-label stimulation phase of the study. In this phase, rDBS was delivered using a bipolar montage of the two ventral contacts on the lead (contacts 1 and 2) on each left and right electrode positioned within the NAc (Fig. 1b). Both subjects received bilateral NAc rDBS via depth electrodes connected to a NeuroPace RNS System to detect and inhibit LOC eating events. Stimulation was delivered at 125 Hz in two 5-s bursts at a charge density of 0.5 μC cm−2. Current was incrementally increased over 6 months in both subjects, resulting in a stimulation charge density of 1.5 μC cm−2. Detections and stimulations occurred approximately 400 times a day with a stimulation limit set to 700 bouts (or approximately 117 min) per day to limit unnecessary stimulation at night (Supplementary Figs. 4.1 and 4.2). The recording phase was used to identify the biomarker that the rDBS would respond to, whereas the stimulation phase was used to test the safety and side effects of stimulation. Stimulation parameters were derived from those used in epilepsy and thought to have a disruptive effect on the target neural activation26,27,28. Stimulation was started at a low amplitude using contacts that a monopolar assessment previously revealed to elevate mood to avoid well-described, DBS-induced side effects to this region such as hypomania. Stimulation was started unilaterally per FDA guidance and added stimulation to the contralateral hemisphere, and then slowly titrated every 3 months until an improvement in LOC was detected. Frequency and pulse width were held constant such that only changes in amperage were made.
Based on the recording phase, each subject’s device was programmed to detect brief increases in low-frequency activity in both the left and the right ventral NAc (Supplementary Information). These detections of low-frequency activity triggered bilateral NAc rDBS (~1 μC cm−2 charge density, 10-s duration). Low-frequency triggered bilateral stimulation has been well tolerated by both subjects. Neither subject 1 nor subject 2 experienced a serious adverse event and all reported events were self-limited (Supplementary Table 1). Examination of sensitivity and specificity can be found in Supplementary Figs. 1 and 2.
High-frequency DBS has been widely used to effectively treat symptoms in psychiatry (for review see Sullivan et al.29). High-frequency stimulation has been found to both excite and suppress neural activation in different ways, and therefore it has been difficult to pinpoint the direct mechanism of action. However, although there are a number of theories surrounding the mechanism of action of high-frequency DBS, only one common mechanism has been found to explain the various neural effects found from high-frequency stimulation: DBS dissociates input and output signals in the stimulated nucleus and disrupts abnormal information flow in pathological conditions (aligning with the ‘disruption hypothesis’ of stimulation)26. Furthermore, therapeutic effect has been linked to effective suppression of low-frequency oscillations, which was accomplished only by high-frequency stimulation17,30.
Data collection and analyses
LFP analyses
All LFP data analyses were performed in R2014b (Mathworks Inc.) using customized scripts built on the FieldTrip Toolbox31 (Donders Institute for Brain, Cognition and Behaviour, Radboud University, the Netherlands; see http://fieldtriptoolbox.org).
Electrophysiological data were recorded at 250 Hz and bipolar re-referenced online. The recorded LFP data were cleaned offline, using the following steps: (1) de-trending raw LFP data; (2) 60-Hz (and harmonics: 120-Hz, 180-Hz) notch filters; and (3) 1- to 90-Hz bandpass filter. The cleaned data were then epoched with respect to the trial onset or, in the case of ambulatory data, centered at the time of the magnet swipe. LFP data recorded during stimulation trials were not analyzed due to the high-frequency nature of the stimulation and associated artifact in the recording.
All spectral analyses were computed at each channel. Data underwent morlet wavelet time frequency transformation and then the power was averaged within the canonical frequencies: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (13–30 Hz), gamma (31–50 Hz) and high-gamma (50–90 Hz) frequency bands. In some cases, we analyzed low-frequency power combining delta–theta (2–8 Hz). Average estimates of bandpower were derived by taking the mean power across the frequencies within each predefined canonical frequency band with the s.e.m. reported. Ambulatory: data epoch was extracted in the pre-magnet swipe time (1–2 min) before undergoing above spectral analysis. Multi-item buffet: data epochs were extracted 2 s before the time-locked bite within each task condition—standard eating and LOC eating. Bites were defined as the moment food entered the subject’s mouth, as defined by visual inspection of synchronized eye tracking (Pupil Lab Pupil Core Eye-tracker (https://pupil-labs.com/products/core)) and video captured during the task. The 2-s windows were chosen based on our previous pre-clinical mouse work looking at the same window with mice approaching the high-fat food5 and examining time-locked eye tracking and video per bite examining time lingering on food in hand/spoon or food coming to mouth. LFP data were captured using the neurostimulator’s magnet feature for storing which was wireless controlled by the research team in an adjacent room with a field of view on the subject behind a one-way mirror window.
Statistical analyses
Statistical analysis (Minitab Data and Statistical Toolbox) was performed using two-sided Student’s t-test to compare bandpower means during clinical tasks. One-way analysis of variance (ANOVA) followed by Student’s t-test was performed comparing mean power during ambulatory recordings under three conditions (control, craving, hunger). The χ2 test was performed on the rDBS detection count for each subject. A statistical significance criterion of α = 0.05 was used for all tests. Results are shown as a mean ± s.e.m. unless otherwise noted.
Signal detection
For each subject, we programmed the device to detect brief increases in low-frequency activity in both the left and right ventral NAc. To help differentiate the brief bursts of low-frequency activity associated with LOC from the longer periods of delta activity observed during sleep, we selected the RNS System ‘Area’ detector. The area under the curve (AUC) detector takes a signal AUC measurement every 2 s (short-term trend) and compares it with an average of AUC measurements from the past 2 min (long-term trend). If the current (short-term) AUC measurement exceeds the average long-term trend measurements of AUC, by the programmed threshold (in our cases by 63–100%), a detection is made. Stimulation detection threshold was determined by the analyses of the abnormal NAc activity. As the low-frequency activity observed during sleep increases the longer-term AUC, it is difficult for the short-term AUC measurement to exceed the longer-term AUC value by the specified threshold, thereby reducing the amount of activity detected during sleep. As we observed increases in low-frequency power during real-world LOC in both the left and the right ventral NAc, we also required the activity to be present simultaneously in both the left and the right NAc for the detection to trigger rDBS delivery.
Clinical measures
The primary study endpoint is at least 50% of subjects exhibiting a decrease in the number of LOC eating events per week, utilizing the Ecological Momentary Assessment (EMA). Reported below are preliminary EMA data from subjects 1 and 2, as well as additional support from the monthly surveys and ELOCS:
(1)
EMA: 1 week out of every month, participants receive a survey at two semi-random times per day (morning and evening) that asks about eating behaviors, including frequency and severity of loss of control. Supplementary Figs. 3.1 and 3.3 display the number of LOC eating events per 28 d, which is the endpoint for the study (any reduction in LOC from baseline), for subjects 1 and 2, respectively.
(2)
ELOCS: a retrospective survey assessed once a month targeting LOC frequency and severity. Supplementary Figs. 3.2 and 3.4 for subjects 1 and 2 display the LOC frequency score and LOC frequency severity based on this 18-item assessment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.